INTRODUCTION
Obstructive sleep apnea syndrome (OSAS) is a disorder in which the throat collapses during sleep, causing apnea or hypopnea. The pathophysiology is multifactorial and includes obesity, craniofacial abnormalities, impairment of pharyngeal muscles and nerves, and fluid shift towards the neck. Consequently, patients present with intermittent hypoxia and hypercapnia, arousals, and an increase in respiratory effort. Symptoms of this disorder include excessive daytime sleepiness, cognitive impairment, mood dysregulation, and asthenia. It is associated with metabolic syndromes, hypertension, cardiac arrhythmias, stroke, coronary heart disease, atherosclerosis, and increased cardiovascular mortality [1]. Continuous positive airway pressure (CPAP) is the current first-line treatment for moderate-to-severe OSAS, and clinical guidelines currently recommend mandibular advancement devices (MAD) as the first-line treatment option in mild and moderate OSAS (apnea–hypopnea index [AHI] <30); meanwhile, for severe OSAS, refuse CPAP [2]. However, several studies have demonstrated the efficacy of MAD in the treatment of severe OSAS, despite its lower extent compared to CPAP. A complete resolution of the disorder, as indicated by an AHI index <5/h or <10/h or a 50% reduction in AHI, is reported in 40%–70% of patients [3,4].
Regarding adherence to MAD treatment, one study has shown that after 12 months, median usage per night was 7.4 h, compared to 6.8 h for CPAP [5]. In addition, many studies have documented that subjective tolerability is superior for MAD compared to CPAP, and the former is preferred by patients and guarantees a better quality of life [6]. MAD appears to be as effective as CPAP in improving excessive daytime sleepiness [7] and blood pressure [5].
Despite this evidence, the option to employ MAD for severe OSAS is limited to a minority of patients who might benefit from preliminary clinical phenotyping to better predict the efficacy of this device.
CASE REPORT
A 52-year-old woman was referred to our sleep unit with suspected OSAS due to snoring and apnea. The patient complained of attention deficit without reporting any excessive daytime sleepiness, fatigue, morning headache, dry mouth, nocturnal choking, or other signs and symptoms of OSAS. She scored 8 points on the Epworth Sleepiness Scale and had a body mass index (BMI) of 20.76 kg/m2. Physical examination revealed retrognathia, absence of tonsils (due to tonsillectomy in childhood), no palatal narrowing, and a grade IV oral cavity in both Friedman and Mallampati classifications.
Video-polysomnography
The patient underwent full-night attended video-polysomnography (v-PSG), which included recording of the following parameters: electroencephalography, electromyogram of the chin and both tibialis anterior muscles, electrocardiogram, electrooculogram, oronasal airflow (nasal cannula), thoracic and abdominal movements, and pulse oximetry. Sleep was scored according to the American Academy of Sleep Medicine guidelines. In the first basal v-PSG, the patient presented with a sleep efficiency of 55.1%, total sleep time of 227.5 min, AHI of 56.2/h (apnea index=24.5/h, hypopnea index=31.6/h, supine AHI= 64.2/h, non-supine AHI=53.7/h, and REM AHI=15/h). All apnea and hypopnea were obstructive. The mean of oxyhemoglobin saturation (SpO2) was 93.3%, the minimum SpO2 was 80.0%, and the percentage of time below 90% SpO2 was 8.5%. Sleep architecture presented: 9.9% N1, 72.1% N2, 5.7% N3, and 12.3% REM with an arousal index of 26.6/h.
Treatment with MAD
Based on the severity, CPAP was proposed for this patient; however, she refused this treatment. She accepted trying MAD as a second therapeutic option. A preliminary ear, nose, and throat evaluation with the Muller maneuver showed a 3rd grade (70%) concentric pharyngeal obstruction, which was more pronounced at the base of the tongue (mainly along the anteroposterior axis) and retrognathia, confirming grade 4 in the Mallampati/Friedman classification and the absence of meaningful palate narrowing.
The dentist’s evaluation documented satisfactory dental and periodontal conditions and good ability to perform protrusive, latero-protrusive, opening, and closing mandibular movements. The patient presented with a class II occlusion (i.e., the mesiobuccal cusp of the maxillary first permanent molar articulates mesial to the buccal groove of the mandibular first permanent molar), with a 7 mm overjet, 3 mm overbite (total excursion, 11 mm), and maximum mouth opening of 42 mm.
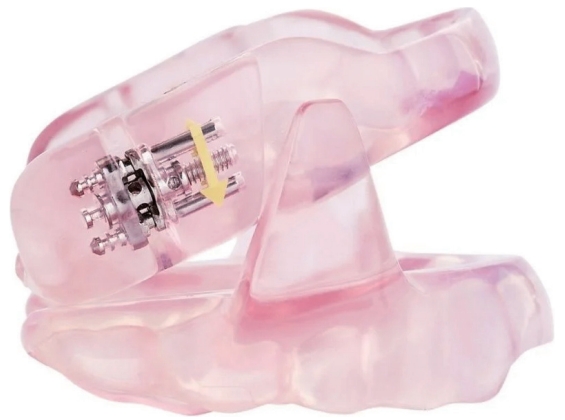
A treatment was started with a MAD Somnodent® Flex (SomnoMed, Plano, TX, USA) (Fig. 1), set to a 7 mm jaw protrusion, representing 70% of the maximum protrusion. In this position, the MAD was optimally titrated, resulting in the resolution of signs and symptoms. The patient reported no complaints in the temporomandibular joints; however, she experienced transitory neck discomfort in the morning during the first two weeks of use. Based on the absence of relevant side effects and targeting the maximal possible efficacy, after two months of treatment, the protrusion was extended to 8 mm, with no further improvement in signs and symptoms or onset of any side effects.
During follow-up visits after three months, there were no complaints related to temporomandibular disorders, masticatory muscles, or alterations in dental occlusion.
Follow-up
Three months later, ambulatory monitoring using cardiorespiratory polygraphy showed a normalization of the AHI (from 56.2/h to 1.3/h), an increase in the SpO2 nadir from 80% to 91%, and an SpO2 <90% of 0% (previous=8.5%). The snoring completely resolved (previous=75.2%). Table 1 shows a comparison of sleep parameters before and after treatment.
The patient reported improvement in sleep quality and quality of life without any side effects attributable to MAD, as already described. Her Epworth Sleepiness Scale score was 5.
DISCUSSION
The patient had severe OSAS, with an AHI of 56.2/h, SpO2 nadir of 80%, and a snoring time of 75.2%. She refused CPAP, which we proposed as a first-line option. After titrating the MAD to 8 mm advancement, a monitoring respiratory polygraphy showed a normalization of sleep breathing with an AHI of 1.3/h, SpO2 nadir of 91%, and a snoring time of 0.2%.
Patients with severe OSAS are at an increased risk of cognitive impairment, daytime sleepiness, mood disorders, and major cardiovascular events [1]. In this context, therapy is mandatory, and CPAP is considered the undisputed first-line treatment, considering its very high efficacy and tolerability compared to other therapeutic options. According to current guidelines, MAD is indicated only for mild and moderate OSAS, and its impact on patients’ general health is minimal [2]. On the other hand, few studies have proven that MAD can also be partially effective in patients with severe OSAS, but to a lesser extent than CPAP [3,4].
Body fat level and anatomical features of the pharynx and neck are crucial for the outcome of MAD treatment. In fact, the response to treatment was linked to a lower BMI, smaller neck circumference, retrognathia, shorter maxillary bone and soft palate, reduced facial height, shorter distance from the hyoid bone to the third cervical vertebra, shorter airway length, and narrower minimum cross-sectional area [8,9]. Moreover, a study that measured fat mass percentage using dual-energy X-ray absorptiometry showed that the neck-to-abdominal fat percentage ratio predicted OSA severity [10].
We believe that the positive outcome was due to the following factors, as indicated by our case report: retrognathia, no palate narrowing, pharyngeal obstruction, mainly along the anterior-posterior axis and at the base of the tongue, wide protrusive mobility of the jaw, and leptosomic appearance (BMI 20.76 kg/m2). Moreover, the female sex is known to be a positive prognostic factor [8].
The outcome was optimal, and the patient only suffered from transitory neck pain at the beginning of the treatment; additionally, until now, she has not shown signs of temporomandibular dysfunction or problems with dental occlusion, which is uncommon, but typical for MAD treatment.
Our investigation was limited because the improvement was evaluated using respiratory polygraphy, which is characterized by inferior sensitivity and specificity compared to PSG performed to establish the diagnosis. However, a critical revision of this exam allows us to state that the flux signal presented almost no reduction during the whole night, even in the supine position, indicating a complete resolution of the disorder, making this exam as valuable as PSG in this specific case.
In conclusion, the MAD resolved the clinical signs and symptoms and improved polygraphic parameters in a patient with severe OSA. There were no side effects, and these results were maintained during a 5 month follow-up. Our patient presented with pharyngeal anatomic and functional features, which may represent a possible positive predictor of outcome in patients with severe OSAS who refuse or do not tolerate CPAP.