Association Between Suggestive Symptom of Restless Legs Syndrome and COVID-19 Vaccination: A Pilot Study
Article information
Abstract
Objectives
Various sensory symptoms have been recognized after COVID-19 vaccination. Here, we aimed to explore the association between the suggestive symptom of restless legs syndrome (RLSss) and COVID-19 vaccination using an online survey.
Methods
We prospectively studied participants who were working in our hospital after at least the first dose of the ChAdOx1 or BNT162b2 mRNA vaccine. The participants were invited via smartphone messages and voluntarily filled out an online questionnaire that included adverse events after vaccination. We considered the participants as having RLSss if they reported that they had three or more symptoms in the restless legs syndrome (RLS) diagnostic criteria.
Results
A total of 628 participants (506 female; mean age, 37.7±12.4 years) responded fully to our online survey. 588 participants (93.6%) received the first dose of the ChAdOx1 vaccine (BNT162b2 mRNA vaccine for 40 participants). A total of 44 out of the 628 participants (7.0%) reported that they had RLSss. Myalgia was more common in participants with RLSss than in those without RLSss (97.7% vs. 67.3%, p<0.001). Multivariate testing showed that age (odds ratio, 1.037 per 1 year increase; 95% CI, 1.004–1.071) and the presence of myalgia (odds ratio, 20.479; 95% CI, 4.266–368.206) were associated with the presence of RLSss.
Conclusions
This pilot study explored RLSss after COVID-19 vaccination and the results suggested that RLS might be one of the causes of adverse symptoms after COVID-19 vaccination. Further studies are required to confirm the relationship between RLS and COVID-19 vaccination.
INTRODUCTION
Globally, national vaccination programs against severe acute respiratory coronavirus-2 (SARS-CoV-2) infection are in place. As the number of vaccinated people increases, safety concerns regarding coronavirus disease 2019 (COVID-19) vaccination have grown. Unexplained complications of COVID-19 vaccination have been revealed as a result of massive vaccination within a given period of time. The ChAdOx1 vaccine has been reported to be related to thrombotic immune thrombocytopenia [1], while the BNT162b2 mRNA and mRNA-1273 vaccinations have recently been reported to be related to myocarditis, and pericarditis [2]. The association between Guillain-Barre syndrome (GBS) and COVID-19 vaccination has also been reported [3], but epidemiological studies have shown no conclusive results [4,5].
In addition to these serious adverse events, common but modest symptoms after COVID-19 vaccination were also investigated; sensory symptoms including localized or generalized soreness, myalgia, itching, and tingling [6,7]. However, clear explanations of these sensory symptoms are largely unavailable, and along with these symptoms, there is also an uncomfortable sensation in the lower extremities. These symptoms were reported to have remarkable similarity with symptoms of restless legs syndrome (RLS) by patients including one of the authors in this study. COVID-19 infection can affect the severity of RLS, but little is known about the relationship between RLS and COVID-19 vaccination [8].
Here, we aimed to explore the association between the suggestive symptoms of RLS and COVID-19 vaccination using an online survey.
METHODS
Participants and procedure
The participants were healthcare employees working at our hospital (Soonchunhyang University Hospital Cheonan). All the participants were invited to participate in our survey via smartphone messages after the first or second dose of the ChAdOx1 or BNT162b2 mRNA vaccine, which were the only available vaccines at the time of study, and were administered according to the distribution protocol in Korea. The participants were informed about the goal and content of the study, and questionnaires were distributed to those who agreed to participate. The study was approved by the local ethics committee and the requirement for informed consent was waived (SCHCA 2021-09-019).
We conducted an online survey using Google Forms. The survey questionnaire included demographic features of the participants, self-reported current height and weight for calculating body mass index (BMI), type of COVID-19 vaccine, and adverse events after vaccination. The suggestive symptoms of RLS were investigated based on the four RLS diagnostic criteria [9]: 1) an urge to move the legs, usually accompanied or caused by uncomfortable and unpleasant sensations in the legs; 2) the urge to move or unpleasant sensations begin or worsen during periods of rest or inactivity such as lying down or sitting; 3) the urge to move or unpleasant sensations are partially or totally relieved by movement, such as walking or stretching, at least as long as the activity continues; and 4) the urge to move or unpleasant sensations are worse in the evening or night than during the day or only occur in the evening or night. The time interval between the onset of symptoms following vaccination and the severity of these symptoms was also evaluated using the visual analog scale (VAS). We considered participants with three or more symptoms from the RLS diagnostic criteria as having suggestive symptom of RLS (RLSss).
Statistical analysis
The demographic characteristics of the participants are presented with appropriate summary statistics. Continuous data are shown as means with standard deviations or medians with interquartile ranges (IQR). Categorical variables were presented as absolute and relative frequencies. We analyzed the differences between the groups (with or without RLSss) using a chi-square test or Fisher’s exact test for categorical variables and Student’s t-test or a Mann-Whitney U test for continuous variables. The Cochrane-Armitage trend test was used to evaluate the proportion of RLSss across the age range. Multivariate ogistic regression analysis was performed to evaluate the independent contribution of the factors that influenced the presence of RLSss. Factors from univariate analyses were considered to represent explanatory variables and were evaluated using multivariate analysis, with the type of vaccine (either ChAdOx1 or BNT162b2 mRNA vaccine), age, sex, BMI, adverse symptoms, and the use of analgesics considered as predictors. Results are presented as odds ratios (ORs) with 95% confidence intervals (CI). Statistical significance was set at p<0.05. All statistical analyses were performed using R Programming software version 4.0.3 the ggplot2 package (R Development Core Team, 2013; http://www.r-project.org).
RESULTS
Demographic features
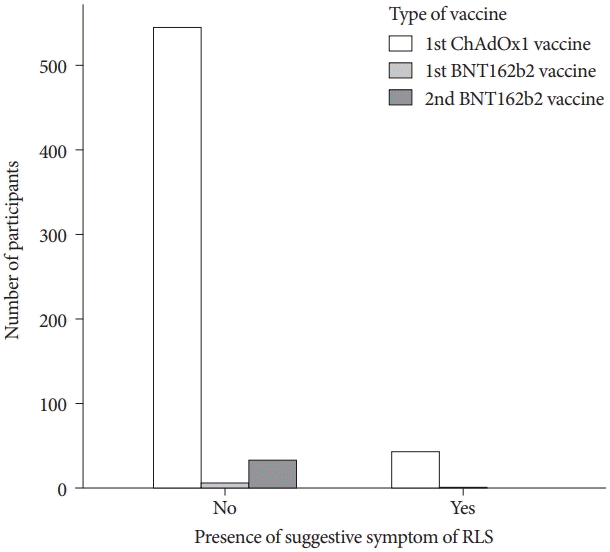
A total of 628 participants completed all questions in the online survey, of which 506 were female (80.6%). Their mean age was 37.7±12.4 years, and their mean BMI was 22.3±3.3 kg/m2. The majority of the participants were vaccinated with the first dose of the ChAdOx1 vaccine (588, 93.6%); only 40 participants were vaccinated with the BNT162b2 mRNA vaccine (first dose, n=7; second dose, n=33) (Fig. 1). Other demographic features of the groups are presented in Table 1.
Characteristics of participants having ‘suggestive symptom of RLS’
A total of 44 of 628 participants (7.0%) were classified as having RLSss with comparable symptoms of RLS. Among those with RLSss, 72.7% (n=32) had no prior experience of symptoms, while 27.3% (n=12) had RLS symptoms previously. The proportion of participants with RLSss tended to increase with age (p=0.235, Cochrane-Armitage trend test) (Fig. 2). Symptoms appeared on the day of vaccination (45.5%) or within 2 days of vaccination (79.5%) in participants with RLSss. In the majority of participants (86.4 %), the symptoms improved within three days of symptom onset. The severity of symptoms, as assessed using the VAS, was 6.5±2.3. The most commonly reported vaccination-related side effects were injection site pain, myalgia, fever, headache, and dizziness. Myalgia was more commonly reported in the participants with RLSss (97.7% vs. 67.3%, p<0.001) (Fig. 3). Most participants (84.7%) chose to take analgesics to relieve various adverse symptoms, including RLSss, and the rate of analgesic use did not differ between the groups. Multivariate testing for independent factors that influenced the presence of RLSss showed that age (OR, 1.037 per 1 year increase; 95% CI, 1.004–1.071) and the presence of myalgia (OR, 20.479; 95% CI, 4.266–368.206) were independently associated with the presence of RLSss (Table 2).

The proportions of ‘RLSss’ according to age groups. There was a tendency that the proportion of participants with RLSss increased according to the age (p=0.235, by CochraneArmitage trend test). RLSss, suggestive symptom of restless legs syndrome.
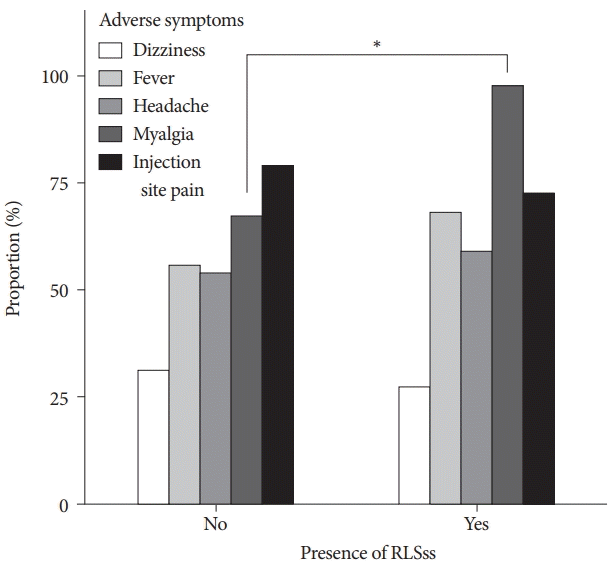
The proportions of adverse symptoms in participants according to the presence of RLSss. *p<0.001. RLSss, suggestive symptom of restless legs syndrome.
DISCUSSION
In this study, we explored the relationship between the RLSss and COVID-19 vaccinations. We found that 7.0% of the participants reported RLSss after COVID-19 vaccination, which was related to older age and the presence of myalgia.
The diagnosis of RLS is based on clinical information, and without objective measurements, diagnostic uncertainty arises for obscure symptoms [10]. Further diagnostic challenges can be investigated for questionnaire-based diagnosis of RLS; however, studies using self-reported questionnaires for the diagnosis of RLS showed comparable results of sensitivity and specificity compared to those of RLS diagnosis using expert interviews [11,12]. Moreover, given the Diagnostic and Statistical Manual of Mental Disorders, 5th Edition (DSM-5) for RLS, short duration of RLSss in our participants may indicate different sensory complaints other than RLS symptoms; DSM-5 criteria include the frequency and duration requirements. However, these variables are not included in the international RLS study group criteria because there could be a risk of underestimating the potential clinical significance of the intermittent subtype or recent-onset RLS [13]. The online questionnaire in our study was based on RLS diagnostic criteria, and it could fairly detect true RLS symptoms.
Although the clinical trials of COVID-19 vaccines did not report major safety concerns, real-world data of COVID-19 vaccination showed various side-effects [14-16]. Commonly reported adverse events include headache, fatigue, fever/chills, diarrhea/nausea, myalgia/arthralgia, and various sensory symptoms [17,18]. Some authors have proposed that these sensory symptoms are caused by an atypical type of GBS involving mainly sensory nerves [19], and others reported that small fiber neuropathy could occur after COVID-19 vaccination.20 While the origins of these sensory symptoms are mostly undiscovered, some sensory symptoms are similar to those of RLS. Furthermore, to the best of our knowledge, RLS-like symptoms associated with COVID-19 vaccination have not been previously studied.
In our study, most participants with RLSss (n=32, 72.7%) had never had such symptoms before, and symptoms resolved within 3 days (n=38, 86.4%). This suggests that unpleasant sensory symptoms following COVID-19 vaccination may suggest a new onset of RLS. The transient characteristics of our participants’ symptoms may suggest that COVID-19 vaccination may have provoked or lowered the threshold for RLS symptoms in participants with subclinical RLS or previous RLS, and most of the participants with previous symptoms of RLS (n=12, 27.3%) also reported that their symptoms were relieved within 3 days (n=9, 75.0%).
The provocation of RLS following COVID-19 vaccination can be attributed to the vaccination itself as well as to weariness, fatigue, or myalgia. Patients diagnosed with RLS commonly report fluctuation of symptoms depending on the exposure to risk factors, such as iron deficiency, medications, or infection [21,22]. It is not clear which was the major contributor to the trigger of RLS symptoms. However, the significant coexistence of myalgia with RLSss, along with short RLSss duration, suggests that acute immune reaction and local inflammation may have played a role. Further studies are required to confirm the relationship between RLS and COVID-19 vaccination.
The clarification of the origins of adverse events after vaccination is important as the experience of unexpected symptoms after vaccination could lead to negative attitudes and reluctant behaviors toward vaccines. The information on safety and reactogenicity profiles of the vaccines could help promote vaccinations and reduce vaccination-related anxiety [23]. With this point of view, the safety and reactogenicity profiles are actively researched in many clinical trials of vaccines, and our study can be a part of this effort to elucidate the origins of adverse symptoms after vaccination. We conducted this study via an online survey using smartphones to collect data from the participants, which could be a useful tool in this COVID-19 era [24]. However, further studies of the correlation between smartphone survey and face-to-face interviews for evaluating RLS are required.
Our study had several limitations. First, we used voluntarily self-reported data via an online survey using a smartphone, which can have potential risks of errors in omission and response bias; some participants might be more inclined to report symptoms than others. Second, because this was a cross-sectional study involving healthcare staff in a single tertiary facility, the demographic profiles of the participants were not controlled, limiting the potential for the generalization of our findings. Finally, there was no control over the type of vaccine used or the timing of the online survey administered after vaccination. However, this was a pilot study, which represents the first effort to clarify the relationship between RLS and COVID-19 vaccination. Further large-scale studies with controlled populations are required.
In conclusion, RLSss can occur after COVID-19 vaccination, and we suggest that RLS might be one of the causes of various adverse symptoms after COVID-19 vaccination. Further studies are required to confirm the relationship between RLS and COVID-19 vaccination.
Notes
The authors have no potential conflicts of interest to disclose.
Author Contributions
Conceptualization: Jin Myoung Seok, Kwang Ik Yang. Data curation: Eun Jin Na, Seul Gi Kim, Jongkyu Park, Eunkyeong Park, Kwang Ik Yang. Formal analysis: Jin Myoung Seok, Kwang Ik Yang. Investigation: Jin Myoung Seok, Kwang Ik Yang. Methodology: Jin Myoung Seok, Kwang Ik Yang. Project administration: Jin Myoung Seok, Eunkyeong Park, Kwang Ik Yang. Supervision: Kwang Ik Yang. Visualization: Jin Myoung Seok. Writing—original draft: Jin Myoung Seok, Kwang Ik Yang. Writing—review & editing: Jin Myoung Seok, Pamela Song, Kwang Ik Yang.
Funding Statement
None.
Acknowledgements
We are very grateful to our colleagues in Soonchunhyang University Hospital Cheonan for their contribution to this study.