Sleep Onset Insomnia and Depression Discourage Patients from Using Positive Airway Pressure
Article information
Trans Abstract
Objectives
Despite the accumulating evidence of the effectiveness of positive airway pressure (PAP) therapy in obstructive sleep apnea (OSA) syndrome, adherence to PAP therapy is not high. Several factors reportedly affect PAP adherence; however, it remains unclear whether patients’ symptoms were detrimental to adherence rate. This study is aimed at investigating the relationship between insomnia symptoms and adherence.
Methods
Retrospective analyses were performed in 359 patients with OSA (mean age 58.4± 13.2 years; females, n=80). Logistic regression analyses were performed between PAP adherence with clinical factors and questionnaires, such as Epworth Sleepiness Scale, Insomnia Severity Index, and Beck Depression Inventory (BDI).
Results
PAP adherence was defined as the use of PAP for ≥4 h per night on 70% of nights during 30 consecutive days. The median follow-up time was 55 days (interquartile range, 30– 119 days), and 54.3% showed poor adherence. Non-adherent patients showed more severe sleep onset insomnia, higher BDI, and higher nadir oxygen saturation (SaO2). Patients with good adherence had higher apnea–hypopnea index, oxygen desaturation index, and respiratory arousal to total arousal ratio. Sleep on-set insomnia [odds ratio (OR)=1.792, p=0.012], BDI (OR = 1.055, p=0.026), and nadir SaO2 (OR=1.043, p=0.040) were independently associated with PAP non-adherence.
Conclusions
Not the severity of insomnia but sleep onset insomnia was associated with PAP adherence, as well as depressive mood. It suggests that different interventions for reducing insomnia and depressive mood are needed to increase PAP adherence in patients with OSA.
Introduction
Obstructive sleep apnea (OSA) is characterized by repetitive upper airway obstruction which leads to nocturnal hypoxia and sleep fragmentation. Positive airway pressure (PAP) therapy stabilizes the vulnerable upper airway during sleep, relieves symptoms, and may prevent or mitigate the adverse outcomes of this condition [1,2]. However, the effectiveness of the treatment is limited by moderate-to-low adherence to PAP therapy [3,4]. Although several studies have focused on factors influencing adherence, no single factor has been consistently identified as predictive of adherence [5-7]. One of the reasons for the discrepancy between studies may be that OSA syndrome is not a uniform condition. OSA is associated with variable degrees of daytime sleepiness and insomnia, suggesting the presence of different clinical phenotypes of OSA [8]. Recently, cluster analysis of the presentation of OSA revealed considerable variations in the presentation as well as different symptom profiles, including disturbed sleep or upper airway symptoms with sleepiness [9]. Another study reported that distinct clinical OSA phenotypes differ in terms of comorbidities and the adherence to nasal PAP therapy [10]. Insomnia, presenting either as a symptom of OSA or independently co-occurring with OSA, was associated with 68% of patients with OSA [11]. The relationships among comorbid insomnia, OSA, and PAP adherence have been evaluated by several studies [11-14]. These studies reported that the severity of insomnia was associated with poor adherence, except for one study found no such association [12]. If insomnia occurs secondary to OSA, treatment of OSA may relieve disturbed sleep. However, if insomnia occurs independently from OSA, it will interfere with PAP treatment. A previous study revealed that although PAP therapy significantly reduced the symptoms of middle insomnia, initial insomnia tended to persist and had a negative effect on adherence [11]. In this regard, not only the severity of insomnia but also type of insomnia (sleep onset insomnia, sleep maintenance insomnia, and early morning awakening) should play a differential role in PAP adherence. This study was aimed at comparing the prevalence of different types of insomnia and their impact on PAP adherence, as well as to identify a predictor of PAP non-adherence that requires more attention.
Methods
Subjects
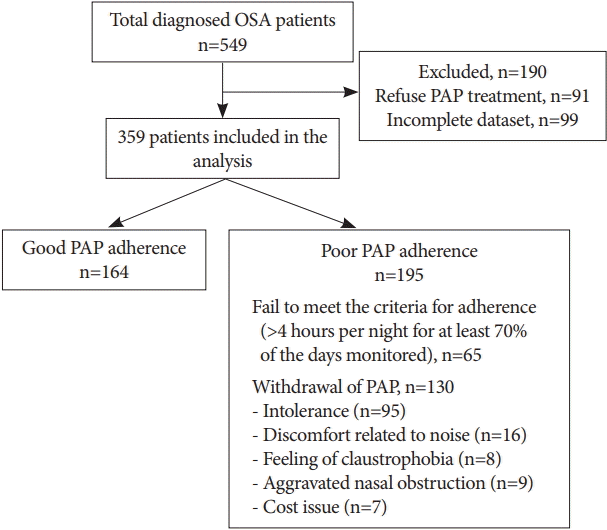
Five-hundred forty nine subjects were diagnosed as having OSA by diagnostic polysomnography (PSG) at a sleep clinic in university hospital between March 2014 and March 2018. Of these, 91 subjects initially refused to use PAP and 99 subjects were excluded due to incomplete data set. Finally, 359 subjects were included in this study (Fig. 1). All the subjects who accepted to use PAP had follow-up appointments set for 1, 3, 6, and 12 months and every 6 months thereafter. This retrospective study protocol was reviewed and approved by the Institutional Review Board at Samsung Medical Center (IRB File No. 2015-02-096-017).
PSG and OSA diagnosis
PSG was conducted using an 18-channel recording system (Remlogic; Embla Co., Broomfield, CO, USA). We used six electroencephalogram channels, two electro-oculogram channels, a four-channel electromyography for chin, intercostal muscles, and right and left anterior tibialis, and channels for nasal flow, arterial oxyhemoglobin saturation, and electrocardiography. PSG records were manually analyzed according to the American Academy of Sleep Medicine (AASM) criteria [15]. Apnea was defined as ≥90% drop of airflow for ≥10 seconds and hypopnea as an air-flow reduction of ≥30% for ≥10 seconds associated 3% oxygen desaturation or an arousal. OSA severity was graded according to the AASM 1998 criteria [16] as follows: mild [apnea–hypopnea index (AHI), 5/h≤AHI<15/h], moderate (15/h≤AHI<30/h), and severe (30/h≤AHI). Oxygen desaturation index (ODI) was the number of times per hour of sleep that the blood oxygen level dropped by >4% from baseline. Apnea–hypopnea ratio shows the extent of upper airway collapsibility. The defined phenotypes were as follows: apnea-predominant phenotype [apnea index (AI): hypopnea index (HI) ratio ≥2], hypopnea-predominant phenotype (AI:HI ratio <0.5), and both apnea–hypopnea-predominant phenotype (AI:HI ratio 0.5–2) [17]. The ratio of respiratory-related arousal to total arousal defined the percentage of respiratory-related arousal (respiratory arousal, snoring arousal, and respiratory effortrelated arousal) among total arousal.
PAP therapy and adherence
PAP initiation
PAP therapy was initiated with home auto-PAP in most instances, except for patients having underlying cardiorespiratory disease or cost issues, for whom a manual PAP titration study was chosen. Overall, 26 patients (14 patients with cost issues, 10 patients with pulmonary disease, and four patients with cardiac disease) were prescribed fixed-pressure PAP after split-night PSG. All patients underwent PAP counseling by a sleep respiratory technologist before the PAP trial. During the session, patients were educated about the benefits and possible side effects of PAP and attended a 30-min maskfitting and equipment education session. Three mask types (full face, nasal, nasal pillow) were available, and every patient could try each mask and choose the one they were most comfortable with. Built-in heated humidification was applied in all cases.
PAP adherence
PAP adherence was defined as >4 h per night for at least 70% of the days monitored [18]. PAP usage information was evaluated from the secure digital card of the device at each visit and included the following variables: prescribed PAP pressure, time since PAP distribution (recorded in days), % of days with >4 h of PAP use, average daily PAP use (min), the mean PAP pressure, the maximum PAP pressure, and the mean residual AHI. Non-adherence to treatment was considered to refer to patients who used PAP less than 4 h/day, stopped using due to intolerance or subjective lack of benefit, or were lost to follow-up.
Self-report questionnaires
The patients filled in a questionnaire regarding sleep-related symptoms, comorbidities (e.g., hypertension, chronic obstructive pulmonary disease, diabetes, cardiovascular disease, neurologic disorder, and other sleep disorders), highest education level attained, medication, smoking history, body mass index (BMI), and the following psychometrics: Epworth Sleepiness Scale (ESS), Insomnia Severity Index (ISI), and Beck Depression Inventory (BDI).
ESS is a self-rating eight-item test, with each item rated on a scale of 0–3 (sum score, 0–24) to verify the subject’s habitual likelihood of dozing or falling asleep. ESS scores over 10 are representative of pathological sleepiness [19]. ISI comprises seven items rated on a scale of 0–4 (none, mild, moderate, severe, very severe, in that order) on a 5-point Likert scale [20]. The first three items of the ISI evaluate the symptoms of “difficulty falling asleep” (ISI 1a), “difficulty maintaining asleep” (ISI 1b), and “problems waking up too early” (ISI 1c). The remaining items evaluate satisfaction with current sleep, degree of daytime impairments, distress, and noticeability of impairments caused by insomnia. Total score categories are subdivided as follows: 0–7=no clinically significant insomnia, 8–14=subthreshold insomnia, 15–21=moderate clinical insomnia, and 22–28=severe clinical insomnia. BDI comprises twenty one questions for measuring the severity of depression (BDI ≥10, clinically significant) [21].
Statistical analyses
Differences in continuous variables between PAP-adherent and -non-adherent groups were evaluated using the unpaired t-test or Mann–Whitney test as appropriate. Categorical data are presented as frequencies with percentages; between-group differences were analyzed using chi square test. Data are reported as mean and standard deviation or median and interquartile range (IQR) as appropriate to test normality. Multivariate analysis was performed using logistic regression modeling with PAP adherence and variables found to be significant at the level of 0.05 in univariate analysis. Variables showing significant differences between the two groups (good vs. poor adherence) were selected and included in the logistic regression analysis. There were possible interaction among variables, such as hypnotic drugs and BDI which could affect the association between insomnia and PAP adherence. Those interactions were also included in the multivariate analysis to test the statistical significance of the interactions. None of the interaction terms reached the 5% statistically significant level. The analysis was performed using SPSS 17.0 (SPSS Inc., Chicago, IL, USA).
Results
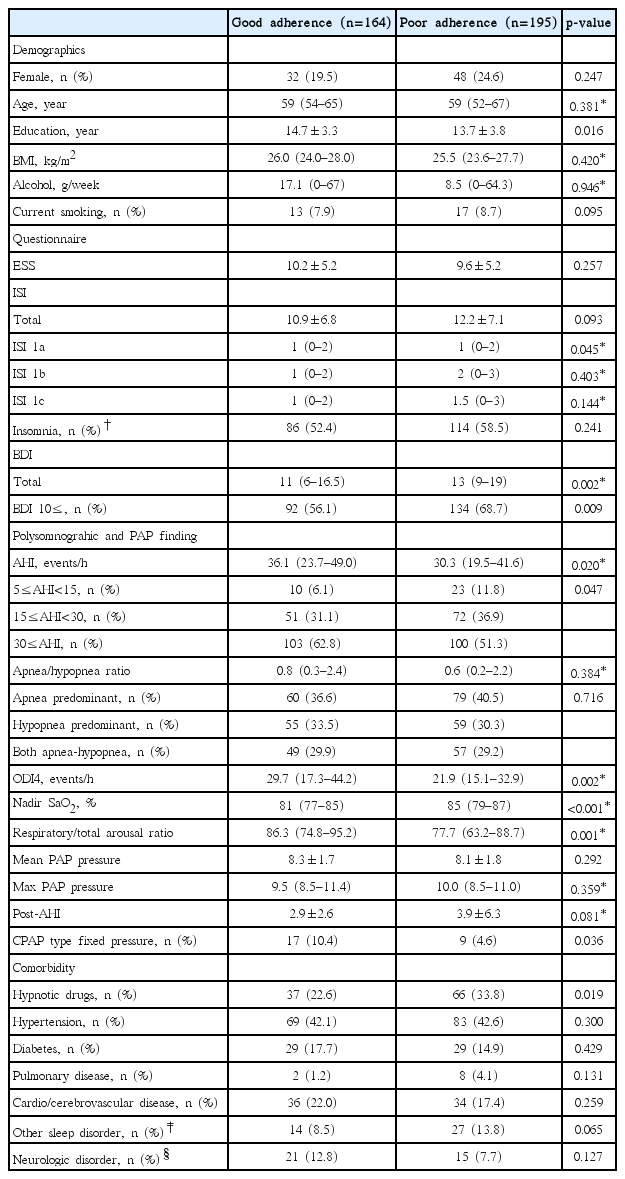
The median observation period was 55 (IQR=30–119, range=11–944) days. Overall, 130 patients (36.2%) withdrew from PAP therapy due to intolerance (n=95), discomfort related to noise (n=16), aggravated nasal obstruction (n=9), feeling of claustrophobia (n=8), and cost issues (n= 7). The dropout rate was 33.7% (n=121), 1.4% (n=5), and 1.1% (n=4) at 1, 3, and 6-month follow-ups, respectively. Although statistically insignificant (p=0.170), OSA severity-related dropout rate [mild, 16/33 (48.5%); moderate, 49/123 (39.8%), severe; 65/203 (32.0%)] showed significant linear by linear association (p=0.013). Among the remaining 229 patients, 65 (18.1%) showed non-adherence despite continued indication for treatment. The overall adherence rate to PAP was 82.1%. Compared with patients with adherence, those without adherence to PAP showed significantly higher ISI 1a, BDI, nadir oxygen saturation (SaO2), and hypnotic drug usage and significantly lower education level, AHI, ODI, respiratory/total arousal ratio, and fixed-pressure PAP usage (Table 1). BMI, ESS, total ISI, current smoking, and comorbidity did not significantly differ between the groups. The variables showing significant differences between the two groups were selected and included in the univariate analysis. Variables significant at the p value of <0.05 in the univariate analysis were considered to represent explanatory variables and variables of interest in this study (e.g., ISI 1a, BDI, nadir SaO2, education, hypnotic drug usage, types of PAP, and respiratory/total arousal ratio); these were further evaluated by multivariate analysis.
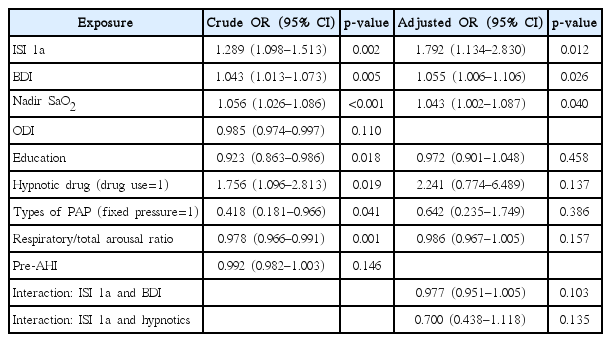
Results from the multivariate logistic regression analysis are presented in Table 2, showing higher ISI 1a [odds ratio (OR)=1.792, 95% confidence interval (CI) 1.134–2.830, p=0.012], higher BDI (OR =1.055, 95% CI 1.006–1.106, p=0.026), and higher nadir SaO2 (OR=1.043, 95% CI 1.002–1.087, p=0.040) in patients with poor adherence.
Discussion
In the present study, the majority (69.8%) of our patients with OSA showed insomnia (total ISI over 8), but the total ISI score was not related with PAP adherence. Instead, sleep onset insomnia affected PAP adherence. The effect of insomnia on PAP adherence already has been studied continuously. Except one study [12], the majority of other studies found that insomnia symptoms and its severity are related with PAP adherence [11,13,14 22,23]. Among those studies, sleep onset insomnia was a significant factor associated with PAP adherence in two studies [11,23]. Björnsdóttir et al. reported the resolution of middle insomnia to an extent in patients with OSA after PAP therapy; however, the symptoms of initial insomnia tended to persist and negatively affected the adherence to PAP therapy [11].
The impact of sleep onset insomnia on PAP adherence could have affected by hypnotic drug usage. However, the usage of hypnotic drug or interaction of the drug on ISI 1a showed no significant effect on PAP adherence from our multivariate analysis. Hypnotic drug seemed to increase the risk of poor adherence in univariate analysis (OR=1.756, p=0.019). However, this does not simply mean the usage of hypnotic drug affected the low PAP adherence, but rather associated with the preceding insomnia requiring hypnotic drugs. Among the studies to identify the usefulness of hypnotic drugs for increasing PAP adherence, two studies showed negative results and one showed that hypnotic drug usage increased PAP adherence [24-26]. Taken together with previous studies and our findings, the efficacy of hypnotic drug for improving PAP adherence seems unclear. Cognitive behavioral therapy has been shown in numerous studies to be effective in improving insomnia [27], also effective in improving PAP adherence of comorbid insomnia and OSA in previous study [28]. This raises the necessity of multidisciplinary therapeutic approach for patients with comorbid insomnia and OSA.
Higher BDI was a predictor of poor PAP adherence (OR=1.055, p=0.026). Depression is associated with poor adherence to medication across a range of chronic diseases [29]. One study reported an independent association between depression and the objective measure of PAP use; however, the study only evaluated the adherence for one week without considering comorbid insomnia [30]. Untreated depression-related fatigue may counteract the improvement of sleep by PAP therapy or depression may sensitize patients to discomfort due to PAP [31,32]. Untreated OSA affects depression negatively, and the depression negatively affects PAP adherence; therefore, treatment of associated depression is important to prevent this vicious cycle [33].
There are studies prove bidirectional interaction between insomnia and depression and they are often comorbid [34]. However, the interaction had no significant influence in our multivariate regression analysis. It is important to note that all of our subjects had OSA which itself is a risk factor for insomnia and depression [35]. So, we presume that OSA may have affected their interaction and hence the analysis as well. From this, we suggest that insomnia and depression are independent comorbid conditions with OSA and targeted therapeutic attention is required to achieve optimal PAP therapeutic outcomes.
The other factor independently associated with PAP adherence was nadir SaO2. A higher nadir SaO2 was associated with a higher risk of poor adherence (OR=1.043, p=0.040). In addition, we showed linear associations wherein the PAP withdrawal rate increased with decreasing severity according to the AHI. Nadir SaO2 and AHI severity were already identified as predictors of PAP adherence in previous studies, probably due to the parameters reflecting the severity of OSA [5,36].
A considerable fraction of patients withdrew using PAP at first follow-up, particularly the patients with mild OSA. This means that we have very short time window of opportunity for PAP adherence support because most patients withdrew PAP therapy even before interventions were made to optimize adherence [37]. In this study, the PAP acceptance rate was 80.2%, which is slightly higher than the previously reported rate of 70% in the Korean population [38]. With recently approved insurance coverage, PAP acceptance is expected to increase. In Japan, lowering the initial cost of PAP increased the acceptance, but the long-term adherence still remains poor [39]. For this reason, effective intervention to improve adherence is essential.
The current study has several limitations. First, we did not assess pre- vs. post-PAP comparisons of ESS, ISI, and BDI. The changes in these indicators before and after PAP could either confirm the therapeutic effect or explain PAP withdrawal due to insufficient therapeutic effect. Secondly, our investigation did not cover socioeconomic status, marital status, and self-efficacy, which are known to be related to adherence [4,23].
Despite these limitations, a key strength of this study is investigating the impact of insomnia on PAP adherence considering specific insomnia phenotypes. In addition, this study discussed various factors known to be associated with PAP adherence and interpreted the results by taking into account the interaction between variables.
In conclusion, we demonstrated that the accompanying sleep onset insomnia, depressive mood, and higher nadir SaO2 were important obstacles to PAP adherence. Therefore, future studies should focus on exploring additional interventions for reducing insomnia and depressive mood for more effective management of OSA.