Circadian Variation of Stroke Onset and Other Clinical Characteristics: A Single-Center Study
Article information
Abstract
Objectives:
This study was performed in order to delineate whether the onset of symptom onset follows circadian rhythm and to identify potential clinical characteristics with similar circadian distribution as that of symptom onset time in stroke patients.
Methods:
We retrospectively analyzed the data of subjects who were registered in a prospectively collected stroke registry in Korea University Medical Center Anam Hospital from September 2007 to December 2013. A Cosinor analysis was used to examine the circadian variation of the symptom onset of stroke as well as various clinical parameters.
Results:
A total of 2,047 patients with stroke were analyzed in our study. The Cosinor analysis showed significant circadian rhythm of stroke occurrence existed according to the symptom onset time of stroke (p<0.001) and the peak time for stroke occurrence was 12:22 (95% confidence interval 11:29–17:41). Cosinor analyses of other variables also showed significant circadian variation of age, fasting glucose, high-density lipoprotein (HDL), and low-density lipoprotein. The circadian distribution of low HDL was similar to that of stroke occurrence.
Conclusions:
The prevalence of stroke occurrence and low level of HDL peaked during the morning.
Introduction
Cardiovascular events tend to occur mostly in the morning.1-3 Stroke is also known to occur most frequently in the morning.4 Although previous studies speculated that circadian rhythm of blood pressure could affect the occurrence of stroke, a recent study showed that there is no significant circadian variation of blood pressure in stroke patients.5 Furthermore, circadian variation of these clinical characteristics in stroke patients might affect prognosis after using tissue plasminogen activator.6 Therefore, the knowledge of circadian variation of various clinical characteristics might help in understanding these differences in prognosis among stroke patients. However, circadian variability in stroke occurrence has not been properly evaluated in Korean stroke patients and there is no systematic studies regarding the circadian variation of clinical characteristics in stroke patients. Therefore, we tried to delineate whether the time of symptom onset in stroke patients follows circadian rhythm using Cosinor analysis and to identify clinical characteristics which show similar circadian distribution as that of symptom onset time in stroke patients.
Methods
Subjects
We retrospectively analyzed the data of subjects who were registered in a prospectively collected stroke registry in Korea University Medical Center Anam Hospital from September 2007 to December 2013. The stroke registry included patients with any kinds of stroke, mostly ischemic stroke, including transient ischemic attack (TIA) who were admitted to the department of neurology at our hospital. For this study, we included only adult patients with age of 18 years old or more at the time of stroke onset. We excluded those patients who did not have any information recorded about the symptom onset of stroke.
Clinical variables
Demographic variables such as age, gender, weight, height, body mass index (BMI), abdominal circumference were included. Systolic and diastolic blood pressures were measured at the time of admission. The etiologies of ischemic stroke were determined according to the Trial of Org 10172 in Acute Stroke Treatment classification: large-artery atherosclerosis (LAA), cardioembolism (CE), small-vessel occlusion (SVO), stroke of other determined etiology (OTHER), and stroke of undetermined etiology (UNDETERMINED).7 Laboratory data such as fasting glucose, hemoglobin A1c, total cholesterol, high-density lipoprotein (HDL), low-density lipoprotein (LDL), triglyceride (TG), and lipoprotein(a) [Lp(a)] were obtained at fasting state in the morning. The symptom onset time was recorded at the registry. The situation at the symptom onset time in stroke patients were classified as 1) ‘during sleep’, 2) ‘upon awakening’, 3) ‘during normal daily activity’, and 4) ‘unknown’. Even if the stroke occurred during sleep or the time of symptom onset was not clear, we tried to estimate the time of stroke using the last normal time. When there was no informant who could give us the information about the symptom onset time of stroke, we recorded the date of symptom onset as the first abnormal time and the time as 00:00. When the symptom onset time of stroke was known, we recorded the symptom onset time up to minutes.
Statistical analyses
A Cosinor analysis was used to examine the circadian variation of the symptom onset of stroke as well as various clinical parameters with the model specified as below:8
where f(t) is a value which we selected to know its circadian rhythmicity, M is the mesor, A is the amplitude, P is the period (in our case, 24 hours), t is the time, and Ф is the acrophase. At first, we analyzed whether the number of patients varies according to circadian rhythm. Then, we analyzed the circadian rhythm of age, weight, height, abdominal circumference, BMI, fasting glucose, hemoglobin A1c, total cholesterol, HDL, LDL, TG, and Lp(a) according to the onset time of stroke. For the variables with similar distribution, Pearson’s correlation analysis was performed. Statistical analysis was performed using R 3.0.3.
Results
Patient characteristics
2,588 patients with stroke were included in this study. The onset time of stroke in 179 patients were not recorded. Although the onset of stroke occurred during sleep in 122 patients and was unclear in 293 patients, we tried to estimate the onset time of stroke using the last normal time. Therefore, we only excluded 362 patients whose symptom onset was exactly at 00:00, since the time of 00:00 was the default time of the symptom onset time for our Stroke Registry program. Among the excluded patients, 13 patients had stroke during sleep and 107 patients had unclear onset. Finally, a total of 2,047 patients with stroke were analyzed in our study. Mean age at stroke onset was 66.9±12.6 years and 61% of the patients were men (Table 1).
Symptom onset time in stroke patients
Stroke occurred most frequently at times of 06:00–12:00 and least frequently at times of 00:00–06:00. Fig. 1A shows the histogram of the symptom onset time. This was also confirmed by Cosinor analysis (Fig. 1B). The Cosinor analysis showed significant circadian rhythm of stroke occurrence existed according to the symptom onset time of stroke (p<0.001) and the peak time for stroke occurrence was 12:22 [95% confidence interval (CI) 11:29–17:41]. Other subgroups according to gender (men and women) and stroke types (TIA, SVO, LAA, CE, OTHER, and UNDETERMINED) all showed significant circadian rhythms with similar distributions.
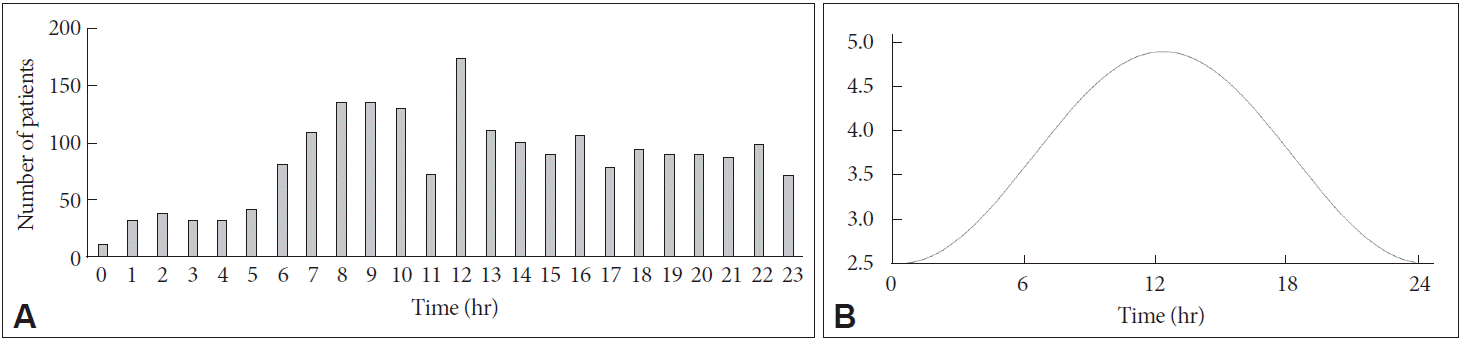
Distribution of symptom onset time in stroke patients. This shows the distribution of stroke patients according to the symptom onset time. (A) The histogram of the symptom onset time shows highest prevalence of stroke onset during early morning. (B) The Cosinor analysis showed significant circadian rhythm of the symptom onset time of stroke (p<0.001) and the peak time for stroke occurrence was 12:22 (95% confidence interval 11:29–17:41).
Circadian variations of other clinical variables
Cosinor analyses of other variables also showed significant circadian variation of age, fasting glucose, HDL, and LDL. The Cosinor analysis showed significant circadian rhythm of age existed according to the symptom onset time of stroke (p=0.036) and the time for the oldest age was 14:52 (95% CI 11:54–18:58) (Fig. 2A). Since age showed circadian variation according stroke onset time, we analyzed the circadian variation of stroke occurrence in older (≥67 years old) and younger (<67 years old) patients. In older patients, the Cosinor analysis showed significant circadian rhythm of stroke occurrence existed according to the symptom onset time of stroke (p< 0.001) and the peak time for stroke occurrence was 12:27 (95% CI 11:23–13:32). In younger patients, the Cosinor analysis showed significant circadian rhythm of stroke occurrence existed according to the symptom onset time of stroke (p<0.001) and the peak time for stroke occurrence was 11:34 (95% CI 10:38–14:38).

Cosinor plot of symptom onset. Cosinor analyses of other variables also showed significant circadian variation of (A) age, (B) fasting glucose, (C) high-density lipoprotein, and (D) low-density lipoprotein. See details in the manuscript.
The Cosinor analysis showed significant circadian rhythm of fasting glucose existed according to the symptom onset time of stroke (p=0.019) and the time for the highest fasting glucose was 20:43 (95% CI 16:30–04:29) (Fig. 2B). The Cosinor analysis showed significant circadian rhythm of HDL existed according to the symptom onset time of stroke (p= 0.007) and the time for the lowest HDL was 13:49 (95% CI 11:10–19:21) (Fig. 2C).
The Cosinor analysis showed significant circadian rhythm of LDL existed according to the symptom onset time of stroke (p=0.040) and the time for the highest LDL was 02:42 (95% CI 23:43–16:22) (Fig. 2D). Other clinical variables did not show significant circadian variation according to the symptom onset time of stroke. Weight (p=0.260), height (p=0.750), abdominal circumference (p=0.123), BMI (p=0.299), systoilic/diastolic blood pressures (p=0.086 and 0.220), hemoglobin A1c (p=0.739), total cholesterol (p=0.057), TG (p=0.071), and Lp(a) (p=0.163) did not have significant circadian variation according the symptom onset time in stroke patients.
Discussion
In this study, we analyzed circadian rhythm of stroke onset as well as that of various clinical factors in stroke patients. The symptom onset time of stroke occurrence showed significant circadian rhythm with peak time of 12:22. Furthermore, age, fasting glucose, HDL, and LDL also showed significant circadian rhythms.
Our study replicated previous studies in that stroke occurred most frequently during the time of 06:00–12:00.4,9-11 For example, a meta-analysis concluded that there is a circadian rhythm in the onset of stroke, that is, increased risk of the onset of acute stroke during the early morning hours.4 This meta-analysis concluded that this finding occurs across the various subtypes of stroke such as ischemic stroke, hemorrhagic stroke, and TIA. We also included all types of the stroke, although most of the stroke was ischemic stroke. This circadian rhythm has also been confirmed by other study with large population up to 1,395 patients.12 It showed significant circadian variation and suggested that circadian variability of blood pressure might be associated with the risk of stroke. However, our result did not reveal the circadian variability of blood pressure.
Cosinor analysis showed the peak time of stroke occurrence as 12:22, which is a little delayed compared to the previous studies. In our study, older patients had delayed stroke occurrence compared to younger patients. Therefore, the delayed occurrence of stroke in our study seems to result from older age of stroke patients in our study compared to the previous studies.4,9-11 The reason for delayed stroke onset time in older stroke patients can be speculated that the disruption of circadian rhythm in elderly patients might play some role. For example, melatonin, which is a primary modulator of circadian rhythms in humans, is also a free radical scavenger and antioxidant and might have some role in neuroprotection for stroke patients.13
In our study, the circadian variation of HDL according to stroke onset time was similar to the distribution of number of stroke patients according to the stroke onset time. Since the HDL is known to have protective role in stroke risk,14-23 similar circadian variation of low HDL and stroke occurrence seems plausible. Since the level of HDL is known to be the lowest in the morning,24 this might explain why patients with low HDL have stroke during the morning. However, the causal relationship between HDL and stroke occurrence cannot be determined in our retrospective study. On the other hand, our study found no circadian variation in the blood pressure as the previous study has noted.5 Although fasting glucose (highest during the evening) and LDL (highest during the dawn) showed significant circadian variation, they do not seem to be related to stroke occurrence.
Previous researchers have suggested several implications on circadian rhythm regarding stroke onset. For example, there were false notion in the past that most strokes occur during sleep,4 which is shown not to be true in the previous studies as well as ours. Since prevention is very important in stroke patients, some risk factors for stroke might be suggested using this kind of analysis.4,12
The limitation of our study was retrospective design. We did not control all possible confounding factors. Our population does not represent a community-based population because our patients were recruited from only neurology department and a single-center hospital. We did not investigate stroke patients according to various stroke subtypes. However, previous study has shown that there were no differences of stroke occurrence according to different subtypes. The population also included recurrent strokes.
