Association Between Sleep and the Metabolic Syndrome Differs Depending on Age
Article information
Abstract
Objectives
Sleep quality and duration are significantly associated with metabolic syndrome (MetS), especially among individuals in the middle-aged and elderly age groups; however, it is inconsistent among the age groups in previous studies. This study investigated the effect of age on the association between sleep and MetS in a large sample size.
Methods
Health-related data on MetS in males and females (n=22,995; age: 23–79 years) were collected from annual health examinations, including the Pittsburgh Sleep Quality Index (PSQI). MetS was diagnosed based on the 2009 Joint Interim Statement.
Results
In total, 4,660 (20.3%) participants were diagnosed with MetS. The data showed a significant association between poor sleep quality (PSQI >5), shorter sleep durations (<6 hours), and MetS in the older group (≥40 years) after adjusting for age, smoking status, alcohol consumption, sex, and exercise. However, this association was not observed in the younger group (<40 years). Regardless of age, global PSQI scores and sleep durations were significantly related to abdominal obesity (male ≥90 cm, female ≥85 cm in waist circumference) and general obesity (body mass index ≥30 kg/m2).
Conclusions
This study demonstrates that sleep quality and duration are related to obesity across all age groups and that sleep quality and duration are only related to MetS in older individuals. These findings suggest that satisfactory sleep in adults aged ≥40 years may play a crucial role in preventing MetS.
INTRODUCTION
Inadequate sleep duration and poor sleep quality are associated with an increased incidence and progression of adverse health outcomes such as cardiovascular disease (CVD) [1,2], type 2 diabetes mellitus (DM) [3], stroke, and cancer [4]. Sleep also has an association with metabolic syndrome (MetS), a cluster of metabolic disorders [5-9]. A meta-analysis has shown a significant cross-sectional association between short sleep duration (< 5 hours) and MetS [6]. Similarly, a prospective cohort study revealed an independent association between short sleep duration (<6 hours) and an increased risk of MetS during an average 2.6-year follow-up [7]. However, the findings from epidemiologic studies on the effects of sleep on MetS are inconsistent [10-12]. For instance, in a study of middle-aged women, there was no significant relationship between sleep disturbances and subsequent incidences of DM during a 35-year follow-up period [10]. In another cohort study of middle-aged (45–64 years) and older (≥ 65 years) subjects, both short and long sleep durations were associated with the prevalence of MetS in middle-aged participants, while only long sleep duration was associated with the prevalence of MetS in older subjects [11]. Certain factors affecting the association between sleep and MetS may explain the discrepancies. In a study on South Korean adults, age was found to be an important effect modifier in the relationship between sleep duration and MetS [12].
The modifying effect of age on the occurrence of MetS In relation to sleep probably stems from the influence of age on sleep and MetS. Sleep quality and quantity change with age because of macro- and micro-sleep architecture changes [13]. Hence, age is inversely related to sleep quality [14]. However, MetS is directly related to age, as previous reports indicated that its prevalence tends to increase with age [15,16].
Thus far, several studies on poor sleep and MetS mostly focused on the association between poor sleep and MetS in middle-aged or elderly people because the prevalence of chronic diseases such as MetS, CVD, type2 DM, and stroke increases with age [8-10], studies on younger individuals (<40 years) are scarce [15]. It is worth noting that early development of MetS has been shown to lead to a higher CVD risk burden [17].
In this study, we hypothesized that sleep duration and quality are related to the prevalence of MetS; however, this association is inconsistent across different age groups. To isolate the specific impact of sleep on metabolic health, it is necessary to investigate the relationship between sleep and metabolic health in two age groups: one before and one after the age at which metabolic health typically begins to deteriorate due to aging-related factors that may affect sleep. Therefore, we investigated the effect of sleep on metabolic health in these two different age groups.
Participants were classified based on age 40, which is known as a time of transition to middle age in a previous study [16,18]. At this point, the incidence rate of chronic diseases, including MetS, begins to increase.
METHODS
Subjects
The study population included participants from the Kangbuk Samsung Health study, a cohort study of adult men and women who underwent a comprehensive annual or biennial examination at the Kangbuk Samsung Hospital Screening Centers in Seoul and Suwon, South Korea [19]. The purpose of the screening program was to promote the early detection of chronic disease and improve health. Because South Korea’s Industrial Safety and Health Law requires that a worker’s employer ensure their employees’ health, and hence employees should participate in annual or biennial health screening examinations. This cross-sectional study included all participants who underwent health examinations, including the Pittsburgh Sleep Quality Index (PSQI), to evaluate their subjective sleep quality and duration between March 2011 and December 2012 (n=27,604). We excluded subjects with a medical history of cancer, psychological diseases, or vascular diseases such as CVD and stroke and those with missing data. Thus, 22,995 participants were included in the final analyses. This study was approved by the Institutional Review Board of Kangbuk Samsung Hospital (KBSMC 2020-03-054), which waived the requirement for informed consent, as we only retrospectively accessed a de-identified database for analysis.
Other measurements
We measured height, weight, waist circumference (WC), and blood pressure as anthropometric data and collected information on the participants’ health behaviors using a wellvalidated questionnaire. We calculated the participants’ body mass index (BMI) (weight in kilograms divided by height in meters squared, kg/m2) using their measured weight and height. We defined central obesity as WC ≥90 cm in males and ≥85 cm in females. According to Asian-specific BMI cutoff points, we classified general obesity into five categories: underweight (BMI <18.5 kg/m2), normal weight (BMI 18.5 to <23 kg/m2), overweight (BMI 23 to <25 kg/m2), obesity (BMI 25 to <30 kg/m2), and severe obesity (BMI ≥30 kg/m2) [20]. We evaluated participants’ smoking status, alcohol consumption habits, and physical exercise levels. The smoking group comprised participants who had smoked more than once during their lifetime, whereas the non-smoking group consisted of participants who had never smoked. To assess the frequency of alcohol consumption per week during the past month, participants were asked, “How often did you drink in a week on average during the past month?”. Physical activity during the past month was evaluated using the question, “How many times a week do you exercise until you sweat?”. Blood samples were (drawn from the antecubital vein) of all participants after more than 10 hours of fasting.
Metabolic syndrome
The presence of each metabolic component was determined using the criteria by the 2009 joint interim statement of the International Diabetes Federation [21]. MetS is characterized by the presence of three or more of the following five components: 1) abdominal obesity, defined as WC ≥90 cm in males and ≥85 cm in females; 2) elevated blood pressure, defined as systolic blood pressure (SBP) ≥130 mmHg and/or diastolic blood pressure (DBP) ≥85 mmHg, or receiving treatment for hypertension; 3) elevated serum fasting blood glucose (FBG), defined as glucose concentrations ≥100 mg/dL or receiving treatment for DM; 4) hypertriglyceridemia, defined as fasting plasma triglycerides ≥150 mg/dL; and 5) low high-density lipoprotein cholesterol (HDL-C), defined as HDL-C <40 mg/dL for males and <50 mg/dL for females or use of dyslipidemic medication.
Assessment of sleep duration and quality
Sleep quality and duration were assessed over a 1-month period using the Korean version of The PSQI [22,23]. The PSQI is a widely used self-reported questionnaire commonly employed by clinicians worldwide to assess several aspects of sleep [22].
The PSQI consists of 19 items, categorized into seven components: subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleep medication, and daytime dysfunction. The sum of the scores for these seven components produced a global sleep quality score ranging from 0–21 points. A global PSQI score of greater than 5 indicates poor subjective sleep quality [22].
Using the results of the sleep duration components of the PSQI questionnaire, we classified the entire study sample into two groups: the short sleep duration (<6 hours) and the long sleep duration (≥6 hours) groups. We used a 6-hour cutoff derived from recent research using polysomnography (PSG), in which sleep duration <6 hours had a detrimental effect on metabolic health [24].
Statistical analyses
Age, sex, smoking status, weekly alcohol consumption, number of days of exercise per week, global PSQI score, sleep duration, and MetS components were compared between individuals with and without MetS. Student’s t-test was used for continuous variables, and the chi-square test was used for categorical variables. We performed a multiple logistic regression analysis to assess the association between the global PSQI score and MetS while controlling for age, sex, alcohol consumption, smoking status, blood pressure, laboratory data, and exercise. In addition, we performed separate stratified multiple logistic analyses in subgroups defined by age (≥40 years, older group; <40 years, younger group) using the same models.
We presented continuous variables as mean±standard deviation (SD), and categorical variables as numbers and percentages. The results are presented as odds ratios (ORs) with 95% confidence intervals (CIs). Statistical significance was set at p-value <0.05. All statistical tests were performed using the SPSS software (version 21.0; IBM Corp., Armonk, NY, USA).
RESULTS
The general characteristics of the participants are presented in Table 1. Of the 22,995 participants, 19,725 (85.8%) were male, and 3,270 (14.2%) were female. The participants’ age ranged from 23–79 years with a (mean±SD, 40.6±7.1 years). The overall prevalence of MetS was 20.3%, which is consistent with the findings of previous studies [15]. Moreover, participants who reported poorer subjective sleep quality (PSQI score >5, 33.4% vs. 30.5%, p<0.001) and shorter sleep duration (<6 hours, 60.6% vs. 56.3%, p<0.001) were significantly more likely to have MetS than those who reported better sleep quality and longer sleep duration (Table 1).
The results of the multivariate logistic regression analysis, which were adjusted for age, sex, weekly alcohol consumption and exercise, smoking status, SBP, DBP, and FBG, are presented in Table 1. The analysis shows that higher PSQI scores, poor sleep quality (PSQI score >5), and shorter sleep duration were positively associated with MetS (OR=1.031; 95% CI, 1.015–1.046; OR=1.183; 95% CI, 1.094–1.279; OR=1.112; 95% CI, 1.032–1.198, respectively). Age-related trends for MetS and participants with poor sleep quality are shown in the Fig. 1 and Supplementary Table 1 in the online-only Data Supplement.
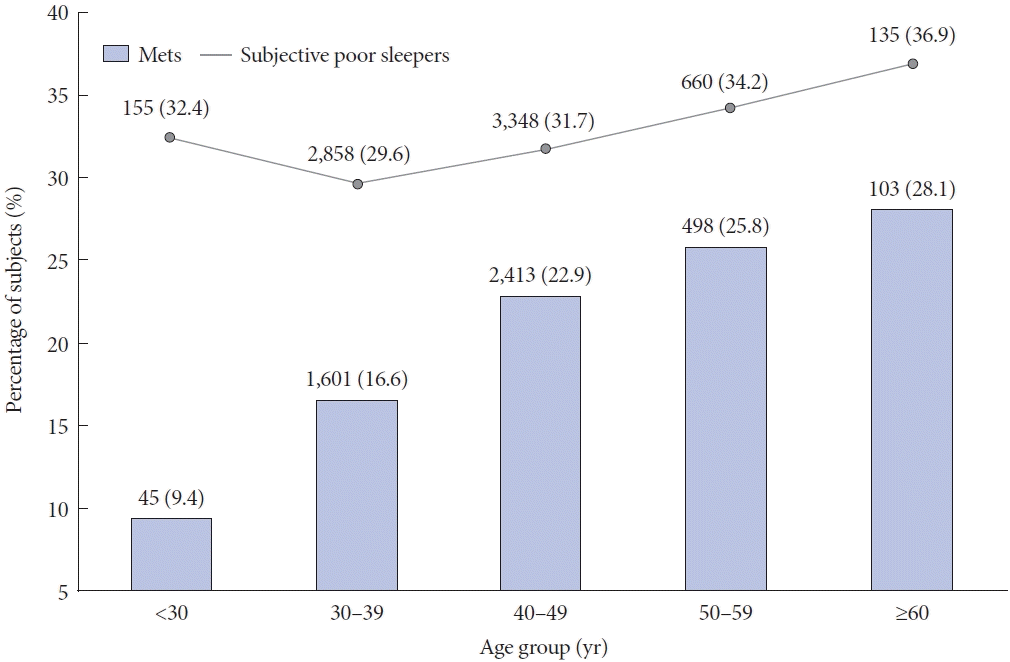
Percentage of participants with metabolic syndrome and poor subjective sleep quality, according to age group. The prevalences of metabolic syndrome and subjective poor sleep quality tend to increase with age in this study. Mets, metabolic syndrome.
The prevalence of MetS has significantly increased since the 40s, which is considered a transitional age of life in Korea, and continued to rise with age, peaking in the oldest age group (≥60 years, 28.1%, p<0.001) (Fig. 1). In addition, poor sleepers showed a slight decrease in prevalence until the 30s, followed by a steady increase from the 40s onwards, and finally peaked in the oldest age group (p<0.001). Furthermore, in addition to MetS and each of its components, drinking and smoking, which can affect the occurrence of MetS, were more prevalent in the older group than in the younger group. However, the number of weekly exercises that could protect against MetS was significantly higher in the older group. All these associations remained significant after adjusting for covariates (Supplementary Table 1 in the online-only Data Supplement).
Subgroup analyses were conducted to evaluate the relationships between MetS and subjective sleep parameters according to age by dividing the groups at a cutoff age of 40 years. The prevalence of MetS was higher in the older group (≥40 years, n=12,847) than in the younger group (<40 years, n=10,148) (23.5%, n=3,014 vs. 16.2%, n=1,646, p<0.001). Furthermore, participants with MetS in the older age group had poorer subjective sleep quality and shorter sleep duration (<6 hours) than those without MetS. This association remained significant after adjusting for potential confounding variables. However, no association between MetS and subjective sleep quality or shorter sleep duration was found in the younger age group (Table 2). Table 2 also shows that the association between poor sleep quality (PSQI score >5) and MetS was found exclusively in the older group.

The Association between presence of metabolic syndrome and subjective sleep quality and duration according to age group
Among the five components of MetS, abdominal obesity was significantly more prevalent in participants with poor sleep quality and shorter sleep duration (Table 3). However, no significant associations were observed between sleep and the other MetS components, except for hypertriglyceridemia, which was associated with poor sleep quality and short sleep duration only in the older age group. Moreover, as shown in Supplementary Table 2 in the online-only Data Supplement, short sleep duration had statistically significant adjusted ORs for BMI categories, including overweight, obese, and severely obese, except for underweight, in both age groups. However, poor sleep quality was significantly associated with severe obesity in both age groups (Supplementary Table 2 in the online-only Data Supplement).
DISCUSSION
This large cohort study classified individuals into two age groups based on the transitional age in the Korean National Screening Program at which chronic disease incidence begins to increase [16,18], that is, 40 years. The study findings demonstrate that the detrimental effects of poor sleep quality and short sleep duration on metabolic health differ with age.
Previous studies have reported that the association between sleep and MetS is moderated by age [11,25,26]. In a cross-sectional study of 2,985 Chinese adults, short sleep duration (≤6 hours) was significantly associated with pre-diabetes among individuals aged ≥60 years, but not in middle-aged (45–60 years) individuals [26]. However, another cross-sectional study has reported that in older individuals (≥65 years), only long sleep duration (≥9 hours) but not short sleep duration (≤6 hours) was linked to a higher prevalence of MetS [11].
This inconsistency among previous studies was the basis of our rationale for our chosen age criterion. Compared to those of previous studies, we used a lower age cutoff. However, as previously mentioned, in Korea, the age of 40 years is recognized as a significant transitional period during when the incidence of many chronic diseases, including MetS, diabetes, and hypertension, begins to increase [16,18]. Moreover, 40% of adults aged >40 years have been reported to have at least two cardiovascular risk factors, such as hypertension, DM, or dyslipidemia [16].
Furthermore, age is an important factor affecting the relationship between sleep and MetS. The prevalence of MetS, sleep quality, and sleep duration changes across the lifespan due to age-related changes in the brainstem and hypothalamic sleep and wake regulatory centers, which result in instabilities in the sleep-wake maintenance systems. Therefore, as people age, there can be instabilities in their sleep patterns that result in shorter sleep durations, longer time needed to fall asleep, and more frequent interruptions to consolidated periods of sleep compared to younger adults [13]. A large, population-based cohort study has shown that the probability of being a “good sleeper,” measured with the PSQI, decreases considerably after the age of 50 years [27]. Our data is consistent with previous studies, as shown in Fig. 1, which shows an increase in the prevalence of subjective poor sleepers with age. Age-related differences in the association between sleep and MetS may occur due to changes in sleep parameters and metabolic health that occur with aging.
Poor sleep quality has been significantly associated with MetS in previous cross-sectional studies and meta-analyses [8,9,28,29]. Similarly, in our study, the prevalence of MetS was significantly associated with poor sleep quality, as measured by the PSQI.
Our study also provided valuable data on the relationship between sleep duration and MetS. We set 6 hours as the standard reference for sleep duration, derived from previous studies [24,30]. In the Penn State Adult Cohort, a study of a random, general population sample, objectively measured short sleep duration (<6 hours) exacerbated the increased risk of all-cause, CVD, and stroke mortality associated with MetS [24]. However, findings on meaningful sleep duration and its relationship with MetS have been inconsistent [6,31,32]. A meta-analysis including 18 studies with a total of 75,657 participants confirmed that a sleep duration of less than 7–8 hours is significantly associated with MetS [6]. In another meta-analysis including predominantly Asian populations, Xi et al. [32] found that only short sleep duration was significantly associated with MetS, while long sleep duration did not have a significant effect. In contrast, another large-scale community-based cross-sectional study that enrolled 133,608 participants found that MetS was associated with both less than 6 hours of sleep and greater than 10 hours of sleep [33]. These discrepancies among previous studies on the effects of sleep duration on MetS may be attributable to different characteristics of the enrolled participants, such as race, age, and comorbidities, or different sleep duration measurement tools used. Some researchers have suggested that long sleep duration might be a consequence of underlying diseases rather than a causative risk factor [34].
In our study, we observed a significant association between MetS and higher PSQI scores and shorter sleep duration (<6 hours) in the older group (≥40 years) but not in the younger group (<40 years). Similarly, in a study using large cohort data from the Nurses’ Health Study II, reported a buffering effect of age on the association between night-shift work and chronic disease risk profiles. Specifically, night-shift work before the age of 25 years was associated with fewer risk factors related to chronic diseases than night-shift work at older ages [35]. We speculate that telomere length (TL) plays an important role in the age buffering effect on the association between sleep and chronic disease. TL, commonly known as a biomarker of aging, contributes to an increase in inflammation [36,37]. A prospective cohort study with a 6-year follow-up confirmed that shortening of this cellular aging marker is associated with a higher metabolic risk profile [38]. Therefore, we assume that older individuals with relatively shorter TL are more vulnerable to poor sleep quality and short sleep duration and that TL shortening might result in MetS. In addition, inadequate and disturbed sleep due to chronic insomnia or sleep-related breathing disorders may contribute to TL shortening [39-41]. However, due to the scarcity of available data, our study did not reveal any effect of insomnia or sleep-related breathing disorders on MetS.
We found that abdominal obesity (male ≥90 cm, female ≥85 cm in WC) (Table 3) and general obesity (BMI ≥30 kg/m2) (Supplementary Table 2 in the online-only Data Supplement) were related to sleep in both age groups of our study. Although our results show that only short sleep duration had a detrimental effect on all ranges of general obesity and that poor sleep quality was only associated with severe obesity, these findings are not different from a previous study [42]. A large number of cross-sectional studies demonstrated that poor sleep quality was significantly associated with only severe obesity, and short sleepers tended to have an increased BMI [42].
Few studies have explored the association between sleep duration and MetS in young adults. However, in one earlier investigation among Korean adolescents aged 12–18 years, sleep duration was not significantly linked with MetS but was significantly associated with general obesity [43]. In another study among 13,742 participants aged ≥20 years in the USA, short sleep duration increased the risk of abdominal obesity, particularly in the 20–39 years age group [44]. We interpret our current results to suggest that the detrimental effect of inadequate sleep results in only abnormalities of anthropometric measures in the younger group. However, these effects are significant enough to cause MetS, which is related to an increased incidence of chronic diseases in older age groups.
This study had several limitations that must be addressed. First, it used a cross-sectional design; therefore, it could not show a causal relationship between sleep and MetS. Age is a known factor associated with both MetS and poor sleep quality and may therefore act as a modifier in their relationship. To minimize this potential confounding effect, we analyzed the subgroups based on rational age criteria in a Korean sample. However, caution should be exercised when generalizing our findings to the Korean population and other clinical settings. Second, only participants with definite sleep disorders were included in the study. However, the risk of MetS associated with short sleep duration was similar in participants with and without insomnia symptoms [31]. As people age, the occurrence of obstructive sleep apnea (OSA), which impairs sleep quality and is associated with MetS [40,41], may increase. Nonetheless, our study did not include data on OSA, which could have affected our results regarding the moderating effect of aging on the association between sleep and MetS. Third, although the PSQI is the most commonly used tool in clinical and research settings, it is a self-reported survey [23]. However, a recent study has indicated that the PSQI global score correlates well with objective sleep quality indices obtained from PSG [45]. Fourth, participants in the current study were healthy young adults who were employed; hence, our results may not be applicable to other populations with chronic diseases. However, our data demonstrating significant associations between subjective sleep quality and duration and MetS, and the effect of age on these associations in a large number of healthy participants, are valuable and relevant for clinical practice.
Conclusion
Our study findings demonstrated that the association between subjective sleep quality and duration and MetS varied by age. These findings indicate that both short sleep duration and poor sleep quality have a greater detrimental effect on older than younger individuals. This effect may be especially important in individuals at an age when chronic disease risk begins to increase.
Future studies are needed to elucidate the effect of age on the association between sleep and MetS. It would be valuable to investigate using objective biomarkers, such as TL, and objective methods for evaluating sleep quality.
Supplementary Materials
The online-only Data Supplement is available with this article at https://doi.org/10.13078/jsm.230001.
Comparisons of clinical characteristics between older and younger groups based on a 40-year-old age threshold
Odds ratio and 95% confidence interval for underweight, overweight, obesity, and severe obesity with respect to normal body weight according to sleep duration and quality
Notes
Kyung Wook Kang and Eun Yeon Joo, contributing editors of the Journal of Sleep Medicine, were not involved in the editorial evaluation or decision to publish this article. All remaining authors have declared no conflicts of interest.
Author Contributions
Conceptualization: Kyung Wook Kang, Eun Yeon Joo, Eun-Jung Rhee. Data curation: Kyung Wook Kang, Eun Yeon Joo, Eun-Jung Rhee. Formal analysis: Kyung Wook Kang, Eun Yeon Joo, Tai-Seung Nam, Won-Ju Park, Kyung Ho Kang. Funding acquisition: Kyung Wook Kang, Myeong-Kyu Kim, Eun Yeon Joo. Investigation: Kyung Wook Kang, Eun Yeon Joo, Eun- Jung Rhee, Hyung Geun Oh. Methodology: Kyung Wook Kang, Eun Yeon Joo, Won-Ju Park. Project administration: Eun Yeon Joo, Eun-Jung Rhee, Myung-Kyu Kim. Resource: Eun-Jung Rhee, Heui-Soo Moon, Hyung Geun Oh. Software: Kyung Wook Kang, Kyung Ho Kang, Tai-Seung Nam, Won-Ju Park. Supervision: Eun Yeon Joo, Myeong-Kyu Kim. Validation: Kyung Wook Kang, Won-Ju Park. Visualization: Kyung Wook Kang, Tai-Seung Nam, Eun Yeon Joo. Writing—original draft: Kyung Wook Kang, Kyung Ho Kang. Writing— review & editing: Eun Yeon Joo, Myeong-Kyu Kim, Eun-Jung Rhee.
Funding Statement
This work was supported by Samsung Medical Center grant (OTC 1190671) and the National Research Foundation of Korea (NRF) grant funded by the Korea government (MIST) (2022R1F1A1064543).


