Air Regurgitation Through the Nasolacrimal Duct During Bilevel Positive Airway Pressure Therapy in a Patient With Obesity Hypoventilation Syndrome
Article information
Abstract
Obesity hypoventilation syndrome (OHS) is defined as the combination of daytime hypercapnia (awake PaCO2 ≥45 mm Hg) and obesity (body mass index ≥30 kg/m2). Untreated OHS is associated with comorbidities, including cardiovascular diseases, heart failure, pulmonary hypertension, and metabolic syndrome. Continuous positive airway pressure (PAP) therapy with non-invasive ventilation is the gold standard for treating OHS. PAP therapy is highly effective; however, some adverse effects can affect long-term compliance. Air leakage through the mouth or around a mask is a common adverse effect of PAP therapy. Air leakage through the nasolacrimal duct or due to unsealed circuits has also been reported as a complication of PAP therapy; however, it is relatively rare. Considering the negative association between the level of air leakage and adherence to PAP therapy, clarifying the cause of air leakage during PAP therapy and minimizing it are key to successful outcomes. We report a case of air leakage through the nasolacrimal duct that was improved by inserting a gel foam patch inside the lacrimal sac of a patient with OHS with a history of reconstructive surgery for nasolacrimal duct obstruction.
INTRODUCTION
Obesity hypoventilation syndrome (OHS) is defined by the combination of daytime hypercapnia (awake PaCO2 ≥45 mm Hg) and obesity (body mass index ≥30 kg/m2) in the absence of other causes of alveolar hypoventilation [1]. Complications of OHS, including cardiovascular, respiratory, and metabolic impairments, can lead to increased morbidity and mortality. Although positive airway pressure (PAP) is considered the first-line treatment for OHS, there is controversy about the preferred modality of PAP therapy for long short- and longterm outcomes [2]. PAP therapy may have adverse effects, including nasal congestion, dry nose, headache, and skin irritation. Air leakage is also a common complaint associated with PAP therapy. There are two types of air leakage: intentional and unintentional. An intentional leakage is intended to rinse the air through holes in the mask or circuit to prevent rebreathing of CO2. Unintentional leakage may be due to an unfit mask and mouth opening, which could lead to dry eyes, dry mouth, disruptive noise, and poor adherence to PAP [3]. Unintentional leakage involving air leakage through the nasolacrimal duct is also uncommon, especially in patients with a history of nasolacrimal surgery or due to unsealed circuits. Here, we describe a case of air leakage into the eye during PAP therapy in a patient with OHS with a history of reconstructive surgery for nasolacrimal duct obstruction.
CASE REPORT
A 62-year-old woman was referred to our sleep clinic with complaints of sleep disturbances, such as snoring, witnessed apnea, excessive daytime sleepiness, exertional dyspnea, and lower-limb swelling. She had hypertension, ischemic heart disease, and diabetes mellitus. Physical examination revealed a weight of 120.1 kg, height of 155.9 cm, and body mass index of 49.4 kg/m2, which indicated morbid obesity. Her lungs were clear and heart sounds were regular. However, she had slight pitting edema in the lower extremities. On arrival, her atrial blood gas showed a pH of 7.345, PaCO2 of 56.6 mm Hg, and PaO2 of 81.7 mm Hg, suggesting mild respiratory acidosis. Laboratory data revealed normal complete blood counts, chemistry, electrolytes, and cardiac enzymes. Chest radiography revealed cardiomegaly. Transthoracic echocardiography revealed left ventricular (LV) dilatation, LV wall thickness, and global hypokinesia with an ejection fraction of 48%. Pulmonary function tests showed the following, indicating a mild restrictive pattern: forced expiratory volume in 1 s (FEV1), 64%; forced vital capacity (FVC), 63%; and FEV1/FVC, 80%. The maximum end-tidal CO2 value while awake was 63.0 mm Hg, suggesting hypoventilation. However, end-tidal CO2 monitoring was unreliable because the patient persistently breathed through the mouth. Based on the daytime hypercapnia and obesity, in addition to LV hypertrophy, global hypokinesia, and mild restrictive patterns, the patient was diagnosed with OHS and underwent split-night polysomnography (PSG). The split-night protocol is performed only when both of the following requirements are met: 1) apnea-hypopnea index (AHI) is 20/h or greater during a minimum of 2 hours on the diagnostic part; and 2) at least 3 hours remain for PAP titration. The initial diagnostic part of split-night PSG identified significant hypoxia with a nadir of 52.1% and an AHI of 71.4/h (mainly hypopneas, 50.2/h) (Fig. 1A). Respiratory events worsened throughout rapid eye movement (REM) sleep, including more frequent and longer events, and greater oxygen desaturation. Considering that the patient already had 64 respiratory events with severe desaturation, we started PAP therapy after approximately 1 h. The continuous PAP (CPAP) titration part of the sleep study was performed at pressures ranging from 4 to 17 cm H2O (Fig. 1B). Despite CPAP treatment, oxygen saturation below 90% lasting 5 min with a nadir of 88% was observed during stable non-REM sleep (Fig. 1C). One week after the initial split-night PSG, the patient was re-titrated with bilevel PAP (BiPAP) from the inspiratory PAP/expiratory PAP of 8/4 to 25/20 cm H2O due to her intolerance to high pressure and persistent hypopneas at a pressure of 17 cm H2O during the titration study (Fig. 2). Throughout the study, the patient frequently complained of air leakage associated with PAP use and eye redness. Although the sleep technician adjusted the position, size, or type of mask to optimize the mask fit, the patient still felt an uncomfortable air leakage. Upon examination, when her nostrils were pressed externally and she performed a Valsalva maneuver, her right eyelid fluttered, indicating air regurgitation into the eye, which occurred due to retrograde passage of air from the nose to the eye through the nasolacrimal duct. Generally, a bubbling test is recommended to confirm a diagnosis of air regurgitation through the nasolacrimal duct. The formation of bubbles in the medial canthus with a few drops of physiological saline was considered positive. However, we did not perform bubbling tests. A re-evaluation of her medical history revealed that a silicone tube was inserted for partial nasolacrimal duct obstruction on the right side. The patient was referred to the otorhinolaryngology clinic for the management of air regurgitation. The patient underwent a procedure in which a small piece of a gel foam patch was inserted inside the lacrimal sac to prevent air regurgitation. At the 1-month follow-up, the patient reported subjective improvement in sleep quality, such as increased total sleep duration, decreased sleep fragmentation, and daytime sleepiness, and no longer complained of dry eye and eye flutter related to air regurgitation through the nasolacrimal duct during BiPAP therapy. The patient also underwent bariatric surgery to treat her underlying disease.
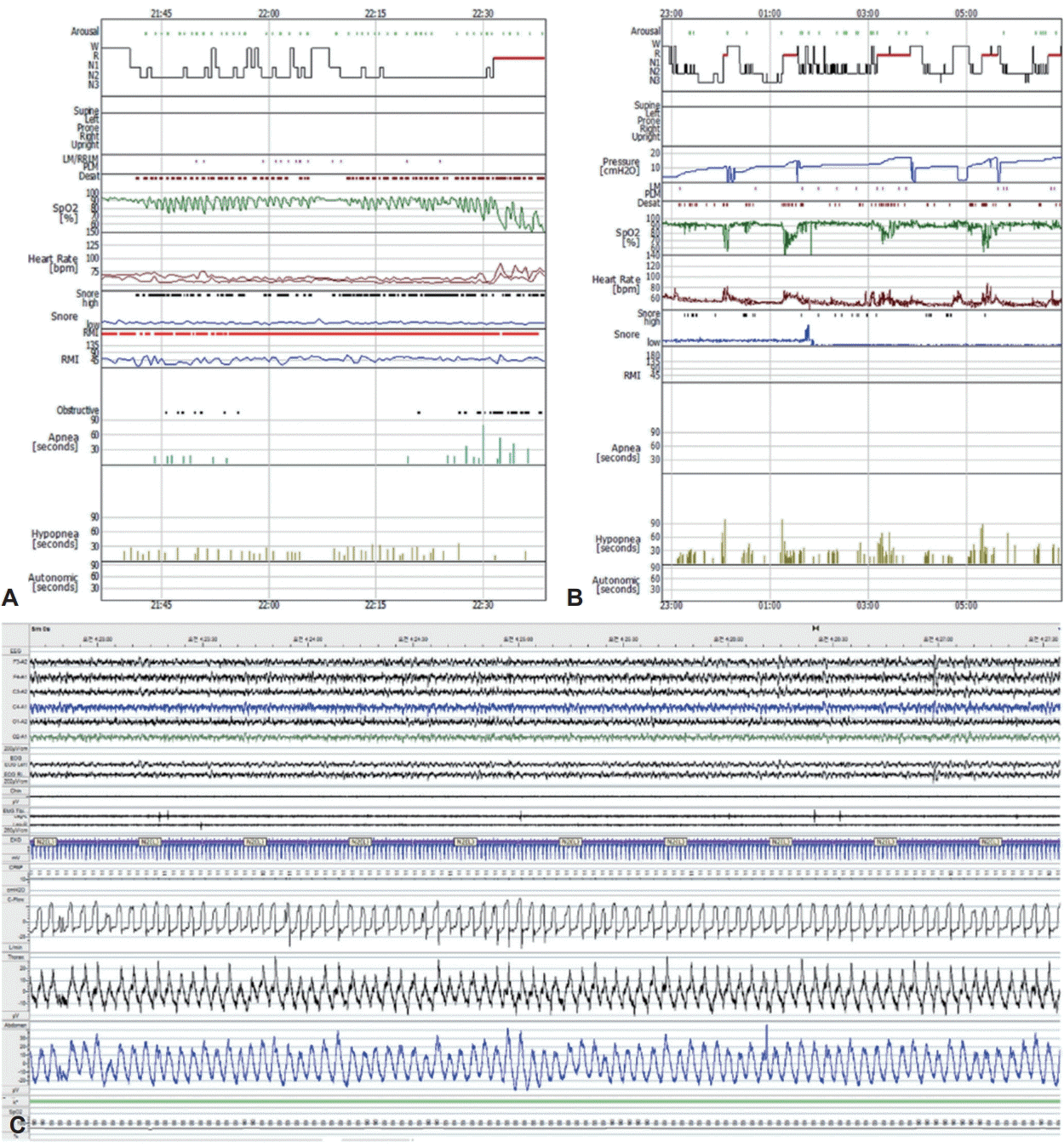
Polysomnographic hypnograms representing severe obstructive sleep apnea and remained respiratory events with hypoventilation during continuous positive airway pressure (CPAP) titration. A: Diagnostic part of split-night polysomnography showing severe respiratory events characterized by aggravation during REM sleep. B: Failed titration with CPAP showing continued respiratory events at 17 cm H2O. C: A 5-min epoch of polysomnography showing oxygen saturation below 90% despite adequate resolution of respiratory events with CPAP.
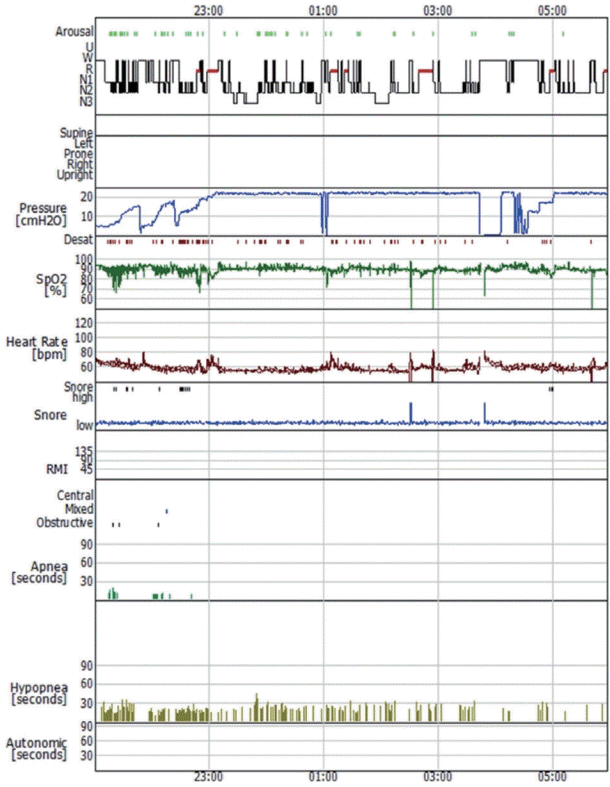
Bilevel positive airway pressure titration showing a trend of decreased respiratory events during the middle third of the study.
After one year, the patient lost 46.6% of her baseline weight from 120.1 kg to 64.1 kg. The follow-up diagnostic part of the split-night PSG showed substantial improvement with an AHI of 34.1/h without apneic events; however, the improvement was modest for the lowest oxygen saturation, which was 66%. A CPAP pressure of 7 cm H2O was sufficient to maintain the airway and prevent respiratory events during titration.
This study was approved by the Institutional Research Board (IRB number: B-2207-769-701). Informed consent was exempt from IRB.
DISCUSSION
Air leakage during PAP therapy is multifactorial and generally originates from the mouth or a mask. Mouth leak is common among patients with nasal obstruction who breathe through their mouth. Mask leaks may result from suboptimally fitted masks related to the headgear being adjusted incorrectly (either too loose or too tight), improper size of mask frame and cushion, micro-tear, and lost elasticity due to old/ damaged mask [4]. Previous studies have shown that air leakage during PAP therapy occurs at the mask interface and can be corrected by changing the mask size and type or by adding heated humidification [5,6]. The nasolacrimal duct is an anatomical structure that connects the lacrimal puncta and nasal cavity and rarely causes air leakage in patients with obstructive sleep apnea (OSA) using PAP. However, dacryocystorhinostomy (DCR), a common and effective treatment for nasolacrimal duct obstruction, is known to increase the incidence of this rare complication, increasing its prevalence to 73% among OSA patients undergoing PAP therapy [7].
The nasolacrimal system plays a key role in maintaining the optimal moisturization of the cornea and conjunctiva. Tear outflow drains from the lacrimal sac to the nasopharynx through the nasolacrimal duct. To prevent backflow, Hasner’s valve, a one-way valve, is located at the distal end of the nasolacrimal duct [8]. Thus, the natural resistance offered by Hasner’s valve may withstand a certain level of PAP; however, as the pressure is increased above the threshold to provide sufficient intraluminal pressure to prevent airway obstruction, this natural resistance may be overcome, leading to air regurgitation through the nasolacrimal duct. Patients with OHS often require highpressure support to achieve adequate tidal volume and improve gas exchange, which could generate more air leakage. Moreover, DCR, a bypass procedure that creates an anastomosis between the lacrimal sac and nasal mucosa through a bony ostium, can introduce an artificial conduit for air leakage and devaluate Hasner’s valve [9]. Previous research has shown that all nasolacrimal systems show air regurgitation after DCR whereas no air regurgitation was observed at CPAP pressures of up to 30 cm H2O in the naïve nasolacrimal system [7]. Furthermore, Blandford et al. [7] suggested that such air leakage could permanently injure the lacrimal canaliculi and puncta and decrease the natural threshold pressure, leading to further aggravation of air regurgitation.
Currently, there is no standard treatment for nasolacrimal duct regurgitation associated with PAP therapy. Using a total face mask [10] or alternative therapy such as the mandibular advancement device [11] has been suggested to be effective. Septoplasty and inferior turbinate outfracture lowering CPAP pressure and reinforcement of Hasner’s valve with nonabsorbable material and injection of a bulking agent around the orifice have also been reported as effective strategies for nasolacrimal duct regurgitation [12]. Our case suggests that a simple minimally invasive technique could be an effective and well-tolerated method for the treatment of nasolacrimal duct regurgitation, even though the patient with OHS required high pressure to improve hypoxemia and hypercapnia.
Although PAP therapy is an effective treatment for OHS, the optimal modality of PAP therapy for OHS has not yet been established. Previous studies have recommended that the adoption of CPAP or BiPAP depends on the OHS phenotype: CPAP for OHS with severe OSA (AHI ≥30) and BiPAP for OHS with no OSA or mild to moderate OSA (AHI <30) [2]. CPAP is preferred for OHS with concomitant severe OSA, whereas BiPAP is the first treatment choice for OHS with pure hypoventilation or fewer obstructive events. If hypoventilation and hypoxia persist despite optimal CPAP, switching from CPAP to BiPAP should be considered. Thus, understanding the underlying pathophysiology is important, and optimal therapy for OHS should not only concentrate on the elimination of obstructive apnea, but also on normalizing PaCO2. Although CPAP was first applied to an OHS patient considering the concomitant severe OSA in our case, CPAP was switched to BiPAP due to intolerance of high pressures on CPAP and persistence of respiratory events.
This is a report of a case of nasolacrimal duct regurgitation, a rare side effect of PAP, in an OHS patient with a history of nasolacrimal surgery. When air leakage is suspected, it is not corrected by adjusting the fit and positions of the masks, and air regurgitation through the nasolacrimal duct should be suspected. This report also suggests that gel foam patch insertion inside the lacrimal sac may be an effective intervention for such leakages.
Notes
The authors have no potential conflicts of interest to disclose.
Author Contributions
Conceptualization: Chang-Ho Yun, Jee-Eun Yoon. Data curation: Seong Kyu Yang, Byeongcheon Lee. Methodology: Jee-Eun Yoon, Dana Oh. Supervision: Chang-Ho Yun, Jee-Eun Yoon. Visualization: Seong Kyu Yang, Byeongcheon Lee, Dana Oh. Writing—original draft: Seong Kyu Yang, JeeEun Yoon. Writing—review & editing: Chang-Ho Yun, Jee-Eun Yoon.
Funding Statement
None
