Sleep related breathing disorders (SRBDs) are common in patients with stroke [1]. However, the relationship between SRBD and stroke is not clear. Central sleep apnea (CSA) is characterized by a loss of central respiratory drive and effort during sleep, resulting in insufficient or absent ventilation, rather than physical upper-airway collapse [2]. CSA is attributed to medical or neurological conditions including stroke. Although brainstem infarctions have previously been suggested to be associated with CSA [3,4], the association of lesion location and CSA in patients with ischemic stroke has not been well elucidated. We report a patient with CSA after acute cerebral infarction.
Case Report
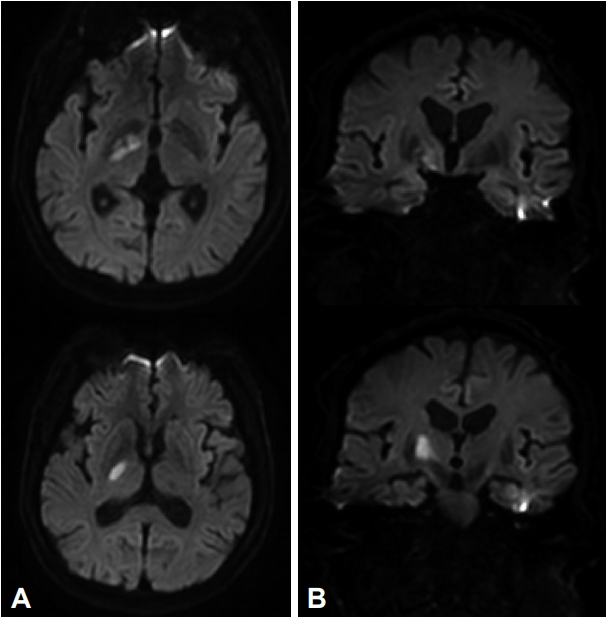
A 69-year-old man with a history of hypertension and diabetes mellitus was admitted to hospital complaining of left hemiparesis, facial palsy, and dysarthria one day prior to admission. Initial National Institutes of Health Stroke Scale (NIHSS) was 7. Magnetic resonance imaging revealed an acute infarction in the right ventral thalamus and adjacent hypothalamus (Fig. 1).
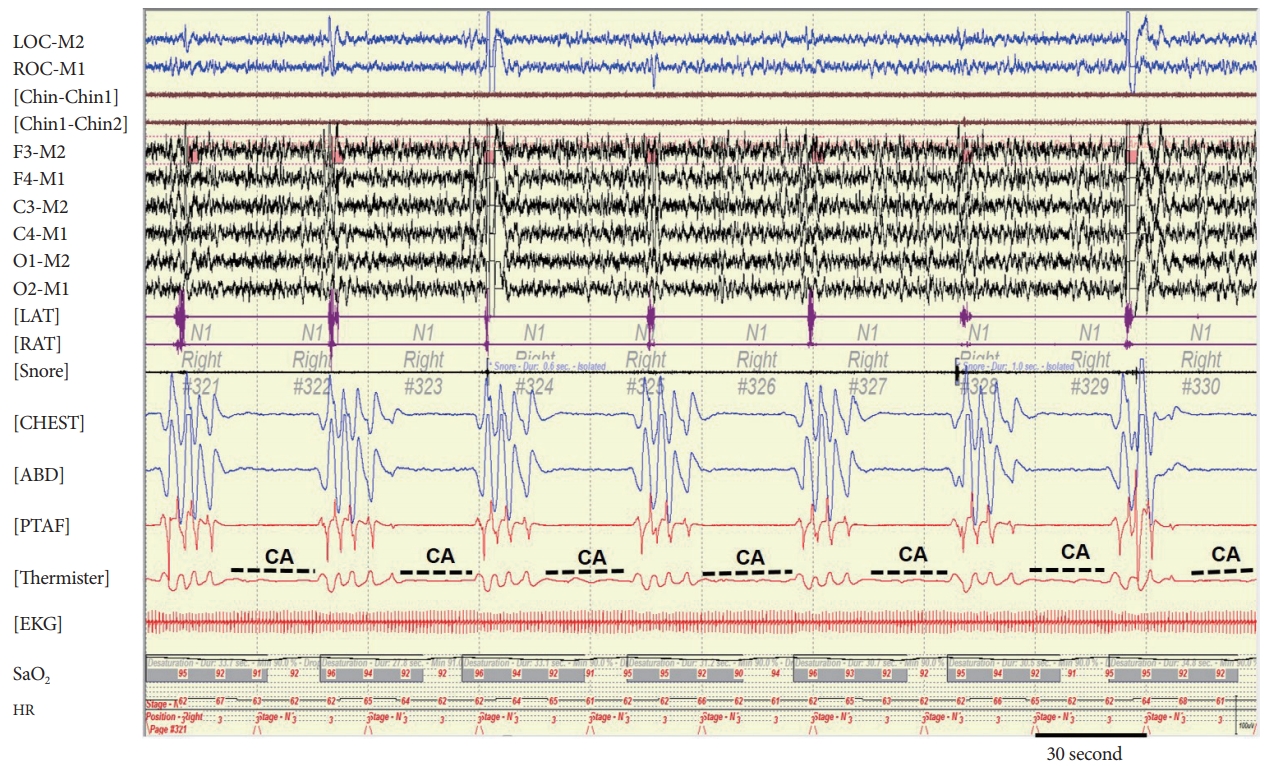
Electrocardiogram, transthoracic echocardiography, transesophageal echocardiography, 24-hours Holter monitoring, and chest X-ray were unremarkable. There was no other history of medical issues and drug use. During hospitalization, repetitive cessation of respiration during sleep was observed by chance. But there was no remarkable finding of respiration when he was wakefulness. He had no suggestive symptoms of snoring and sleep apnea before the stroke. Polysomnography (PSG) (COMET PSG; Twin 4.5.2 Software, Grass Technologies, Warwick, RI, USA) was performed, which showed severe CSA with 324 respiratory events consisting of 295 central apneas, and 29 obstructive hypopneas. The events of CSA were not associated with Cheyne-Stokes breathing (CSB). The overall apnea-hypopnea index (AHI) was 73.5 with a minimum oxygen saturation of 89%. Central apnea index (CAI) was 63.0 (Fig. 2). The respiratory events were not associated with significant oxygen desaturation below 88%. So, nocturnal non-invasive ventilation such as adaptive servo-ventilation was not applied. Two years later, the patient had no sequelae of stroke and follow up PSG showed that AHI was 7.2 with a minimum oxygen saturation of 91% and CAI was 1.0 (Table 1).
Discussion
Although obstructive apnea is more common, central apnea may be noted initially after stroke. It is difficult to determine the relationship between prior and post-stroke SRBD in case of no prior objective diagnosis of SRBD [2]. Although our case did not have PSG finding before stroke onset, it is reasonable to assume that CSA caused by acute ischemic stroke because it resolved spontaneously over two years. The CSA following a stroke is abrupt in onset and tend to resolve with time [5]. However, well-illustrated PSG finding showing typical CSA without CSB has rarely been reported. On this case, the CAI was normalized and markedly reduced compared to initial PSG (63.0 vs. 1.0).
This case showed acute ischemic lesion in the right ventral thalamus and adjacent hypothalamus. Post-stroke sleep-disordered breathing has been well introduced but no specific lesion location was related to the SRBD as well as CSA [6]. Previous studies showed that brainstem stroke caused CSB [4] and high prevalence of CSA without CSB [3]. In a few additional reports, various lesions such as cortical and subcortical area have been reported to be associated with central periodic breathing during sleep in acute ischemic stroke [7]. The mechanism of CSA after stroke has been discussed to be a direct consequence of the injury of central nervous system structures, which is involving autonomic and volitional respiratory centers [8,9]. Within the brainstem, the medulla oblongata contains central chemoreceptors that response to a loop-gain feedback system on the PaCO2 [10]. Lesions to this area lead to decrease chemosensitivity during sleep and develop CSA. Besides, non-brainstem regions might have role of autonomic and voluntary respiration involving insular, and supplementary motor cortex and thalamus [8,9]. It is also known that some hypothalamic nuclei are interconnected with respiratory nuclei located in the brainstem and involve respiratory control as well as other autonomic brain function. Although dysfunction of hypothalamus causes abnormal breathing, the role of the hypothalamus in regulation of respiration during sleep remain to be elucidated.11 Similarly, it is not well known yet about specific anatomical structures and pathomechanism related to CSA after stroke.
This case demonstrates an example of CSA without CSB after ischemic stroke with right thalamus and adjacent hypothalamus, which resolved spontaneously with time. Further elucidation of mechanisms how these structures regulates involuntary respiratory function during sleep is required.