INTRODUCTION
Obesity hypoventilation syndrome (OHS) is a sleep-related hypoventilation disorder defined as the presence of obesity (body mass index >30 kg/m2) with chronic daytime hypercapnia (PaCO2 ≥45 mm Hg) in patients without any other causes to explain alveolar hypoventilation [1,2]. The diagnosis of OHS is important, given the clinical aggravation leading to respiratory dysfunction along with the mortality rate in underdiagnosed patients [3]. Also, OHS has been rising and is now a relatively common cause of chronic respiratory failure with hypercapnia, as obesity rates continue to increase. Therefore, an accurate diagnosis and therapeutic approach for OHS are necessary. We report a case of hypoxic brain damage in a patient with OHS who was treated with bi-level positive airway pressure (BiPAP) therapy.
CASE REPORT
An obese 78-year-old female was admitted to the neurological department because of acute mental deterioration. A nursing assistant checked whether she had slept the night before and found that she was lying in bed, with vomiting and unconsciousness, the following morning. She had a history of hypertension, diabetes, and mild cognitive impairment. She could barely walk and was assisted by a walker as she was both overweight and aging. There was no history of infection or intestinal disease. She had no respiratory problems, had never smoked, and usually did not suffer any related symptoms, such as dyspnea. Physical examination revealed a height of 160 cm, weighed of 81.0 kg, and body mass index of 31.6 kg/m2, indicating obesity. On neurological examination at arrival in the emergency department using an oxygen mask with 15 L/min flow, her consciousness level was a semi-coma status, with a Glasgow Coma Scale (E2, M4, V1) of 7. Furthermore, her brain stem reflexes were normal without other neurological deficits. The initial arterial blood gas test under oxygenation showed severe respiratory acidosis and hypercapnia (pH 7.111, HCO3 38.8 mEq/L, PaCO2 120.5 mm Hg, PaO2 130.6 mm Hg, and SaO2 97.1%), and airway intubation and mechanical ventilation were performed. There were no specific findings in the blood tests performed (including ammonia, infection marker, glucose, and thyroid hormone levels) to differentiate the various encephalopathies.
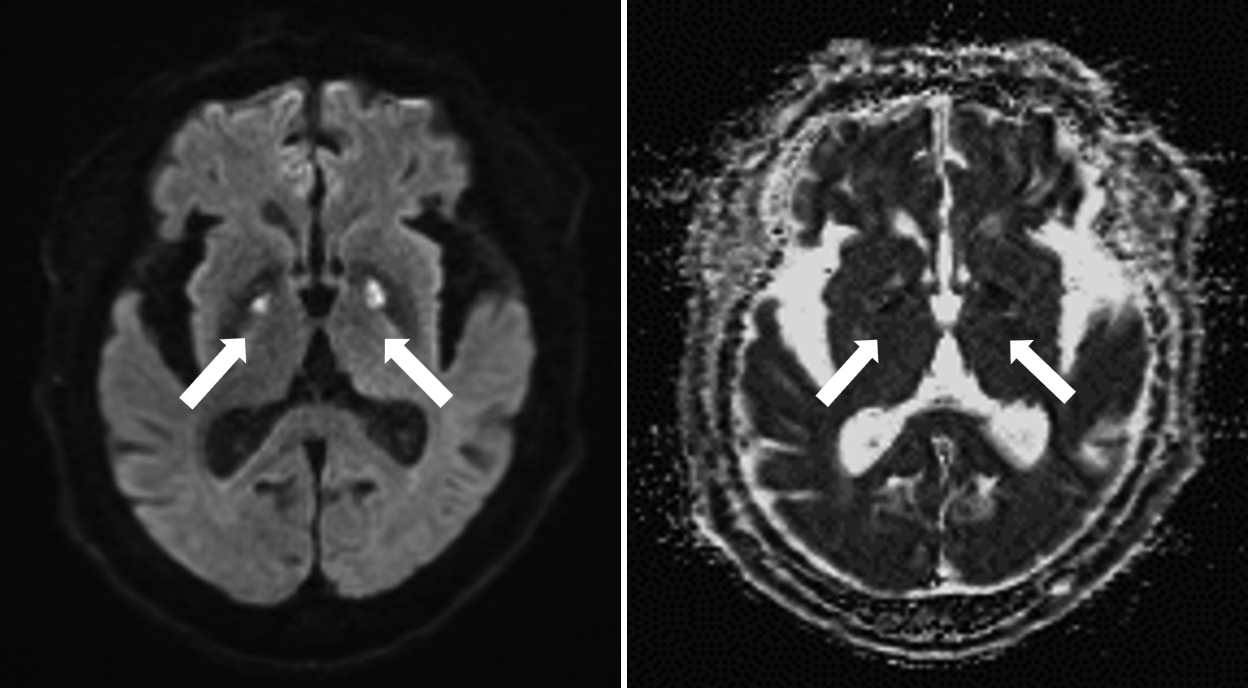
Following mechanical ventilation, her hypercapnia improved within three hours of arrival at the hospital, and her mental state recovered within five hours. Diffusion-weighted brain magnetic resonance imaging (MRI) performed on the fifth day after admission showed acute restricted lesions in the bilateral basal ganglia, indicating hypoxic brain damage (Fig. 1). Although the patient was clearly alert, a mechanical ventilator was applied for 12 days, because CO2 retention was not adequately resolved. There was no evidence of aspiration pneumonia on initial chest computed tomography (CT) with contrast, but prophylactic antibiotics were administered for 12 days, during ventilator application. The results of the arterial blood gas test after the ventilator removal still showed mild respiratory acidosis (a pH of 7.389, HCO3 29.9 mEq/L, PaCO2 49.2 mm Hg, PaO2 75.0 mm Hg, and SaO2 94.5%).
In the other exploration of respiratory failure, there were no anatomical explanations for hypoventilation or findings suggestive of heart failure, pulmonary hypertension, and pulmonary thromboembolism through various tests, such as echocardiography, pulmonary thromboembolism-CT, and bronchoscopy. However, the patient still showed hypercapnia upon waking in the morning (peak PaCO2 56.3 mm Hg), and this value was higher than that in her awakened state during the daytime (peak PaCO2 48 mm Hg). A pulmonary function test was performed two days after the removal of mechanical ventilation in a patient-cooperative state and showed a severe restrictive pattern: the forced expiratory volume in one second/ forced vital capacity ratio (80%) was greater than normal, and the forced vital capacity (37%) was low, but there were no clear pulmonary diseases, such as chronic obstructive pulmonary disease (COPD) or asthma. Despite preserving her alertness, the patient showed excessive daytime sleepiness and snoring. She was usually very sleepy during the day and fell asleep while speaking. Cognitive impairment was confirmed through a mini-mental state examination score of nine. We suspected not only sleep breathing disorder, but also OHS as her diagnosis based on findings of hypercapnia, particularly during early morning and nighttime, along with a history of obesity. On day 19 following admission, a split-night polysomnography (PSG) study was performed. The PSG revealed severe obstructive sleep apnea (OSA), with a high apnea-hypopnea index (AHI) of 60.2/h (mainly a hypopnea index of 59.0/h). The PSG (Fig. 2) also showed sleep-aggravated hypercapnia and nocturnal hypoxemia during rapid eye movement sleep, with hypopnea events. In addition, the maximum end-tidal carbon dioxide values were 51 mm Hg during awakening and 61 mm Hg during sleep. The continuous positive airway pressure (CPAP) was adjusted by increasing the pressure from 4 to 15 cm H2O, but hypoventilation and hypercapnia still occurred, leading to BiPAP titration, even during limited night hours. During titration, the optimal BiPAP pressure was determined, with an increased inspiratory pressure of 18 cm H2O and expiratory pressure of 12 cm H2O at a nadir AHI of 2/h. After three months of BiPAP use, the arterial CO2 retention in the morning was lowered, from 55.4 mm Hg to 38 mm Hg, and the residual AHI was 4.1/h. She adhered to BiPAP therapy for an average of 7 hours and 17 minutes, with 99% usage during the prescribed days. The patient is being followed-up on an outpatient basis, with no clinical signs of dyspnea, edema, nor neurological symptoms, including daytime sleepiness and cognitive impairment. Her family members subjectively believe that communication with the patient is smoother than before, and her cognitive decline improved, despite the lack of objective cognition or sleepiness measures. In summary, we report a case of OHS combined with OSA that presented with acute exacerbation of chronic respiratory failure and hypoxic brain damage. Furthermore, the patient’s condition improved after the BiPAP treatment. The patient provided written informed consent.
DISCUSSION
OHS is characterized by obesity and daytime hypercapnia that occurs in the absence of an explanation for hypoventilation [4]. Previous studies have shown significant health impairments in these patients. They often endure prolonged periods of hospitalization and draw heavily on healthcare resources, with worsening comorbidities and high mortality [3-5].
Patients with OHS suffer from OSA and exhibit severe and prolonged oxygen desaturation during sleep, which results from reduced lung volumes and increased airway resistance [6]. Therefore, hypoxia in OHS is sustained, and notably worsens during sleep.
Prior studies have reported that patients with chronic respiratory insufficiency, such as COPD, are susceptible to hypoxic brain damage and cognitive dysfunction, including increased inflammation and oxidative and physiological stress [7]. Congenital central hypoventilation syndrome is a disorder that affects hypoventilation during sleep, resulting in a shortage of oxygen and a buildup of carbon dioxide in the blood. This potentially leads to absent or reduced ventilatory and arousal responses to sustained hypercapnia and, to a lesser extent, sustained hypoxia [8]. Some studies have revealed that several brain regions in patients with congenital central hypoventilation syndrome respond inappropriately to problems with ventilation or blood pressure. In prior study results, the brain lesions typically appeared in the forebrain diencephalon, midbrain, and cerebellar areas [9]. MRI findings of hypoxic brain damage preferentially indicate lesions in areas such as the cerebellar hemispheres, basal ganglia, or cerebral cortex [10]. The patient in our presenting case exhibited hypoxia by asphyxiation from vomiting, following accelerated aggravated hypercapnia on OHS. Given the clinical history and circumstances of the present case, hypoxic injury may be a convincing explanation for the pathophysiology of brain injury in the patient rather than other infectious, vascular, epileptic, genetic/congenital, metabolic, or toxic causes.
Chronic hypoventilation in patients with OHS exposes them to chronic hypoxia and daytime hypercapnia, which leads to an accumulated hypoxic burden and susceptibility to even minimal levels of hypoxic insult. OHS often remains underdiagnosed in clinical practice and presents with a lack of specific symptoms or tools, such as in-lab PSG and carbon dioxide measurement [1]. It is important for clinicians to be aware of and suspect the possibility of OHS, given that early detection and proper management are critical for improving patient quality of life and even averting mortality. Therefore, physicians must consider early ventilatory management to provide neuroprotection against delayed hypoxic brain damage during the acute phase in patients with OHS who have experienced a hypoxic event, and even in those without definite respiratory failure.
Patients with OHS combined with severe OSA should be treated with CPAP or BiPAP to manage sleep-disordered breathing and improve nocturnal gas exchange [6]. However, high CPAP was insufficient for resolving the hypercapnia and hypopnea in this patient. Therefore, we adjusted the BiPAP to provide pressure support and improve hypoventilation. This case study suggests that patients with OHS may be susceptible to anoxic damage. Notably, we would like to highlight the importance of concerns surrounding OHS in obese patients and introduce successful treatment with non-invasive ventilation in order to prevent chronic respiratory failure.